- EN - English
- CN - 中文
Nerve Preparation and Recordings for Pharmacological Tests of Sensory and Nociceptive Fiber Conduction Ex Vivo
药理学测试中感觉和伤害感受神经纤维传导的离体神经准备与记录
发布: 2024年04月05日第14卷第7期 DOI: 10.21769/BioProtoc.4969 浏览次数: 1882
评审: Willy R Carrasquel-UrsulaezSalim GasmiMarco Pagliusi Jr.
Abstract
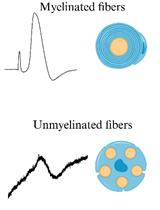
Measuring signal propagation through nerves is a classical electrophysiological technique established decades ago to evaluate sensory and motor functions in the nervous system. The whole-nerve preparation provides a valuable model to investigate nerve function ex vivo; however, it requires specific knowledge to ensure successful and stable measurements. Although the methodology for sciatic nerve recordings has long existed, a method for reliable and long-lasting recordings from myelinated and non-myelinated (nociceptive) fibers still needs to be adapted for pharmacological testing. This protocol takes benefits from epineurium sheath removal for pharmacological tests and provides a detailed description of how to make accurate nerve preparations, from the dissection and handling of nerves to epineurium cleaning, fabrication of adaptable suction electrodes for appropriate fiber stimulation and recordings, setting of electrophysiological protocols for compound action potential (CAP) recordings to distinguish between myelinated and non-myelinated (nociceptive) fibers, and finally to the analysis of the datasets of CAP components. We also demonstrate the feasibility of CAP recordings from individual branches in epineurium-free nerve preparations and provide clues to help retain nerve viability and maintain stable recordings over time. Although a sciatic nerve preparation was used here, the methodology can be applied to other nerve-type preparations.
Key features
• Detailed and simplified protocol for peripheral nerve preparation for recording sensory inputs ex vivo.
• Recordings from myelinated and non-myelinated (nociceptive) fibers can be performed hours after nerve preparation.
• The protocol involves the epineurium removal to facilitate drug permeability into nerve tissue for pharmacological tests.
• The protocol allows physiological and pathological studies (pain/chronic pain conditions).
Graphical overview
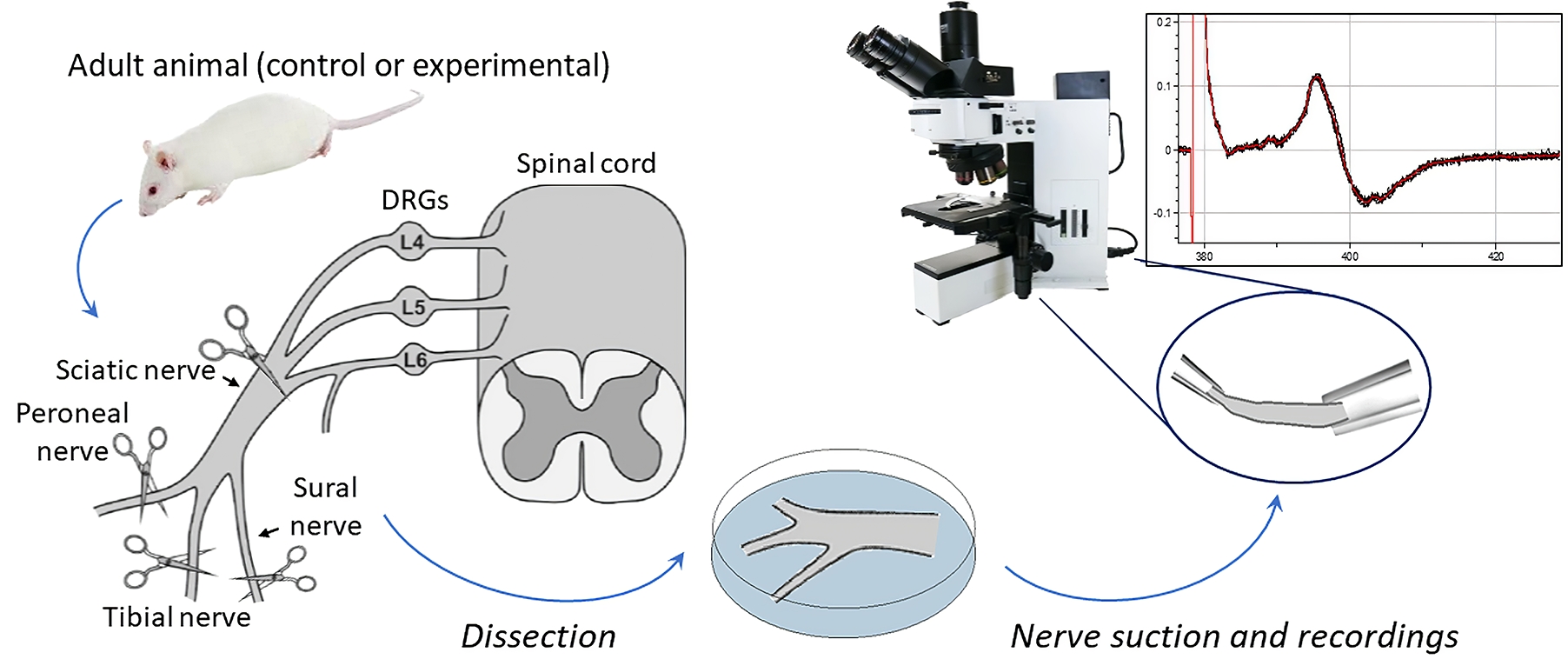
Preparation and recordings from the sciatic nerve, including myelinated and non-myelinated (nociceptive) fibers
Background
It is estimated that more than 1.5 billion people worldwide suffer from pain, with hundreds of millions experiencing chronic pain that remains untreated. As a result, there is a need for new therapeutic options to be developed and new therapeutics are actively being searched [1]. Although there is consensus that a wide range of ex vivo nervous system models provide a valuable tool for studying pain and the mechanisms of nervous system pathologies, whole-nerve models are the only ones that can directly assess nerve conduction in both physiological and pathological conditions. The sciatic nerve is the largest and longest nerve in the body, making it the preferred nerve for many peripheral nerve models [2]. Despite the variety of rodent models that have been established in the past for in vivo and ex vivo recordings [3–7], the methodology for sciatic nerve recordings remains challenging and requires a detailed step-by-step description. Furthermore, detailed protocols for long-lasting readouts from both myelinated and non-myelinated (nociceptive) fibers, especially in the context of pharmacological testing, are still lacking. Stable and reliable recordings are critical to determine the effectiveness of any treatment being tested. This protocol provides a step-by-step description of how to accurately make reliable nerve preparations from rodents, which can be used to monitor nerve conduction over an extended period of time, in physiological conditions, and under pharmacological treatment. The distinctive feature of our protocol is nerve preparation with epineural sheath removal to facilitate pharmacological tests, including recordings from individual nerve branches. We describe electrophysiological protocols for recording compound action potential (CAP), which reflects action potentials (APs) generated by peripheral fibers, both afferents and efferents. In a bundle of nerve fibers, each fiber generates APs, resulting in a current flowing through the surface of the nerve bundle, which has high resistance and can be measured as a potential difference, i.e., CAP, using two electrodes placed on the nerve.
The protocol results in accurate measurements from myelinated and non-myelinated (nociceptive) fibers for a few hours. Additionally, we have demonstrated the effect of lidocaine, a widely used classical local anesthetic, on the nerve as a proof-of-concept to validate the model’s applicability for testing pharmacological treatment.
Materials and reagents
Biological materials
Sprague-Dowley rats 3–6 months old (Charles River, strain code: 001)
Reagents
Glucose (Sigma-Aldrich, catalog number: G8270)
Sodium chloride (Sigma-Aldrich, catalog number: S9888)
Sodium bicarbonate (Sigma-Aldrich, catalog number: S0751)
Sodium monophosphate (Sigma-Aldrich, catalog number: S6040)
Potassium chloride (Sigma-Aldrich, catalog number: P9333)
Magnesium chloride (Sigma-Aldrich, catalog number: M2670)
Calcium chloride (Sigma-Aldrich, catalog number: C7902)
Solutions
Krebs bicarbonate solution (see Recipes)
Recipes
Krebs bicarbonate solution (final volume 0.5 L in ddH2O)
Reagent Final concentration (mM) Quantity or Volume Sodium chloride 125 3,653 mg Glucose 10 901 mg Sodium bicarbonate 26 1,092 mg Sodium monophosphate 1.25 75 mg Potassium chloride 2.5 93 mg Magnesium chloride 1 102 mg Calcium chloride 2 147 mg Total n/a 500 mL Note: To avoid precipitation, the solution should be bubbled with 95% O2 and 5% CO2 before adding calcium chloride.
Laboratory supplies
Isoflurane (Abbvie, catalog number: B506; also, Henry Schein, catalog number: 1182097 or any available)
Spray bottle with 70% ethanol (Fisher Scientific, catalog number: BP82031GAL)
Petri dish 100 mm × 15 mm (Corning, catalog number: 351029)
Glass capillaries with filament O.D. 1.5 mm, I.D. 0.86 mm (e.g., Sutter Instruments, catalog number: BF-150-86-10 or Harvard Apparatus, catalog number: GC150F-10)
Butane blow torch
95% O2 and 5% CO2 gas mixture
Equipment
Dissection and preparation
Anesthesia induction chamber (VetEquip, US) or any other available
Standard scissors (Fine Science Tools, catalog number: 14002-12)
Blunt surgical scissors (Fine Science Tools, product number: 14003-12). Alternatively, hemostat (Fine Science Tools, catalog number: 13003-10)
Coarse forceps (Fine Science Tools, catalog number: 11652-10)
Spring scissors (Fine Science Tools, catalog number: 15025-10)
Fine forceps (Fine Science Tools, catalog number: 11412-11)
Fine forceps (Fine Science Tools, catalog number: 11413-11)
Cordless trimmer for rodents (Bioseb, model: Bio-1584)
Electrophysiology and visualization
Stereomicroscope (Olympus, model: SZX7)
Light source (AmScope, model: 6 W LED Dual Gooseneck Illuminator or any available)
Upright microscope (Olympus, model: BX50WI)
4× objective (Olympus, PLN)
Camera (any equipped with the microscope)
Patch clamp amplifier with headstage (Molecular Devices, model: Multiclamp 700B)
Digitizer (Molecular Devices, model: Digidata 1440)
Micromanipulators (Scientifica, model: PatchStar)
Constant current stimulator (Digitimer, model: DS3)
Pipette holders (Molecular Devices, model: 1-HL-U)
Faraday cage (Sutter Instruments, model: AT-36FC)
Silver wire AWG 26 for electrodes (WPI, catalog number: AGW1510)
Ag/AgCl pellets (WPI, catalog number: EP1)
BNC cables
PC computer
Software and datasets
pClamp (version 10.7, Molecular Devices)
Clampfit (version 10.7, Molecular Devices)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Krotov, V. and Kopach, O. (2024). Nerve Preparation and Recordings for Pharmacological Tests of Sensory and Nociceptive Fiber Conduction Ex Vivo. Bio-protocol 14(7): e4969. DOI: 10.21769/BioProtoc.4969.
分类
神经科学 > 周围神经系统 > 坐骨神经
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link