- EN - English
- CN - 中文
Proximity Labelling to Quantify Kv7.4 and Dynein Protein Interaction in Freshly Isolated Rat Vascular Smooth Muscle Cells
利用近端连接分析在新鲜分离的大鼠血管平滑肌细胞中定量Kv7.4与Dynein蛋白的相互作用
发布: 2024年03月20日第14卷第6期 DOI: 10.21769/BioProtoc.4961 浏览次数: 1562
评审: Pilar Villacampa AlcubierreWilliam C. W. ChenAnonymous reviewer(s)
Abstract
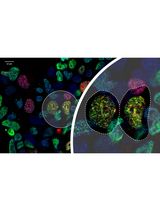
Understanding protein–protein interactions is crucial for unravelling subcellular protein distribution, contributing to our understanding of cellular organisation. Moreover, interaction studies can reveal insights into the mechanisms that cover protein trafficking within cells. Although various techniques such as Förster resonance energy transfer (FRET), co-immunoprecipitation, and fluorescence microscopy are commonly employed to detect protein interactions, their limitations have led to more advanced techniques such as the in situ proximity ligation assay (PLA) for spatial co-localisation analysis. The PLA technique, specifically employed in fixed cells and tissues, utilises species-specific secondary PLA probes linked to DNA oligonucleotides. When proteins are within 40 nm of each other, the DNA oligonucleotides on the probes interact, facilitating circular DNA formation through ligation. Rolling-circle amplification then produces DNA circles linked to the PLA probe. Fluorescently labelled oligonucleotides hybridise to the circles, generating detectable signals for precise co-localisation analysis. We employed PLA to examine the co-localisation of dynein with the Kv7.4 channel protein in isolated vascular smooth muscle cells from rat mesenteric arteries. This method enabled us to investigate whether Kv7.4 channels interact with dynein, thereby providing evidence of their retrograde transport by the microtubule network. Our findings illustrate that PLA is a valuable tool for studying potential novel protein interactions with dynein, and the quantifiable approach offers insights into whether these interactions are changed in disease.
Keywords: Proximity ligation assay (近端连接分析)Background
Studying protein–protein interactions is crucial for unravelling the subcellular distribution of proteins, contributing to our understanding of cellular organisation. Besides revealing the spatial arrangement of proteins, interaction or co-localisation studies provide insights into the functional relationships between proteins and cellular processes. These studies play a role in clarifying signalling pathways, as the interaction partners of certain proteins often indicate their involvement in specific signalling cascades. Additionally, co-localisation studies can contribute to the elucidation of mechanisms governing the trafficking of proteins within cells. For example, identifying whether a certain protein interacts with the microtubule network or specific motor proteins, such as kinesin or dynein that are responsible for cargo movement along the microtubule network in opposite directions, provides insights into trafficking dynamics, including the direction of trafficking.
Various techniques exist for detecting protein–protein interactions in different cellular contexts, including live and fixed cells, tissues, and protein lysates. These techniques typically involve the use of antibodies or direct fluorescent protein fusions. Förster resonance energy transfer (FRET) is a well-established technique for measuring co-localisation in live cells by expressing two proteins of interest, each fused to different fluorophores (donor and acceptor) [1,2]. Proximity of the two proteins induces energy transfer between the fluorophores, which can be measured. However, FRET has limitations, requiring cultured cells and molecular cloning for the fluorophore fusion to proteins. Co-immunoprecipitation, another method for studying protein–protein interactions, involves using antibodies to pull out targeted proteins from cell or tissue lysates, along with some of the direct interacting partners [3]. Analysis using western blotting or mass spectrometry can identify the binding partners of the targeted protein. However, the success of co-immunoprecipitation can be affected by the choice of lysis buffer since this can easily disrupt several protein–protein interactions. Thus, this technique is not optimal for low-affinity interactions. Additionally, co-immunoprecipitation lacks detailed information about the spatial co-localisation of proteins within a cell. Fluorescence microscopy is a widely used technique, which involves labelling proteins with specific antibodies and fluorescent labels, with co-localisation assessed by evaluating the spatial overlap of two fluorescent signals. Although this technique does not detect direct physical protein–protein interactions, advanced light microscopy techniques, such as stimulated emission depletion or structured illumination microscopy, enhance the resolution, providing a more precise determination of protein co-distribution.
The in situ proximity ligation assay (PLA) enables accurate, spatial detection of protein–protein interactions in fixed cells and tissues [4]. This technique involves using two primary antibodies for the targeted proteins, raised in different species. The species-specific secondary PLA probes, each linked to a unique DNA oligonucleotide, bind to their respective primary antibodies. When the targeted proteins are within 40 nm of each other, the DNA oligonucleotides on the PLA probes interact and serve as a template for the hybridising connector oligonucleotide, resulting in the formation of circular DNA through enzymatic ligation. This circular DNA template undergoes rolling circle amplification, producing DNA circles linked to the PLA probe. Fluorescently labelled oligonucleotides are added, which hybridise to the complementary circle DNA. The resulting fluorescent spots can be visualised using fluorescent microscopy. These detected signals can be quantified and attributed to specific subcellular locations, to determine the co-localisation pattern within cells. Overall, PLA offers a precise way to study the spatial relationships between proteins in cells. Moreover, PLA enables the application of pharmacological treatments to isolated vascular smooth muscle cells prior to fixation, allowing exploration of treatment impacts on the co-localisation of specific proteins. In our study, we employed PLA to visualise the interaction of the motor protein dynein with voltage-gated potassium Kv7.4 channels in freshly isolated vascular smooth muscle cells from rat mesenteric arteries [5]. Our goal was to explore dynein's impact on the microtubule-dependent trafficking of Kv7.4 channels. The Kv7.4 and Kv7.5 channels are important regulators of the resting membrane potential in smooth muscle cells, particularly from arteries [6]. Additionally, these channels contribute to the β-adrenoceptor-mediated vasodilatation in vascular smooth muscle cells [7] and other cAMP and cGMP-derived relaxations [8,9]. In arteries from hypertensive rodents, the Kv7.4 channel is downregulated and function is attenuated [10], which contributes to the reduced β-adrenoceptor-mediated vasodilatation observed in these arteries [11]. Our previous studies indicate that dynein motor proteins facilitate the trafficking of Kv7.4 channels along the microtubule network, away from the plasma membrane, in rat vascular smooth muscle cells [5,12]. Furthermore, manipulating this trafficking system by inhibiting the dynein-mediated trafficking of Kv7.4 channels enhances Kv7.4 membrane abundance, restoring the β-adrenoceptor-mediated vasodilatation in arteries from hypertensive rats [13].
Materials and reagents
Biological materials
Rat (Janvier Labs, France)
This protocol has been employed on male and female rats, including strains of Wistar Kyoto, Wistar Hannover, and Spontaneously Hypertensive rats, aged between 10 and 18 weeks. It is anticipated that this protocol is applicable to rats of other strains and ages.
Reagents
Milli-Q water
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888), stored at room temperature
Sodium gluconate (C6H11NaO7) (Sigma-Aldrich, catalog number: S2054), stored at room temperature
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: 12636), stored at room temperature
Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670), stored at room temperature
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C1016), stored at room temperature
Glucose (C6H12O6) (Sigma-Aldrich, catalog number: G7021), stored at room temperature
HEPES (Sigma-Aldrich, catalog number: H3375), stored at room temperature
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9647), stored at 4 °C
1,4-dithiothreitol (DTT) (Sigma-Aldrich, catalog number: DTT-RO), stored at 4 °C
Papain from papaya latex (Sigma-Aldrich, catalog number: P4762), stored at -20 °C
Collagenase from Clostridium histolyticum Type F (Collagenase F) (Sigma-Aldrich, catalog number: C7926), stored at -20 °C
Collagenase from Clostridium histolyticum Type H (Collagenase H) (Sigma-Aldrich, catalog number: C8051), stored at -20 °C
Triton-X (Sigma-Aldrich, catalog number: T8787), stored at room temperature
4% paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: HT501128), stored at 4 °C
Phosphate buffered saline (PBS) (Gibco, catalog number: 10010023, or any other PBS without magnesium and calcium), stored at room temperature
Rabbit anti-Kv7.4 antibody (Abcam, catalog number: ab65797), stored at -20 °C
Mouse anti-Dynein antibody (Abcam, catalog number: ab23905), stored at -20 °C
Duolink in situ PLA probe anti-mouse PLUS (Sigma-Aldrich, catalog number: DUO92001-100RXN), stored at 4 °C
Duolink in situ PLA probe anti-rabbit MINUS (Sigma-Aldrich, catalog number: DUO92005-100RXN), stored at 4 °C
Duolink in situ detection reagents red, with 5× ligation buffer and5× amplification buffer (Sigma-Aldrich, catalog number: DUO92008-100RXN), stored at -20 °C
Duolink in situ wash buffer A and wash buffer B (Sigma-Aldrich, catalog number: DUO82049-4L), stored at room temperature or at 4 °C once dissolved (see Recipes)
Anti-dynein (Mouse; 1:500: ab23905; Abcam) and anti-Kv7.4 (Rabbit; 1:200; ab65797; Abcam)
Calcium chloride (1 M) (see Recipes)
Calcium chloride (100 mM) (see Recipes)
Magnesium chloride (1 M) (see Recipes)
HEPES-Krebs solution (see Recipes)
Smooth muscle cell dissection solution (SMDS) (see Recipes)
Stock solutions (see Recipes)
Tube 1 (see Recipes)
Tube 2 (see Recipes)
Recipes
Prepare all solutions in volumetric flasks up to the specified volume. Using volumetric flasks will contribute to precise measurements and reliable results in your experiments.
Calcium chloride (1 M)
Reagent Final concentration Quantity or Volume Calcium chloride 1 M 11.098 g Dissolve the calcium chloride in Milli-Q water, add the volume up to exactly 100 mL, and store at 4 °C.
Calcium chloride (100 mM)
Dilute 100 μL of calcium chloride 1 M in 900 μL of Milli-Q water and store in a 1.5 mL tube at room temperature.
Magnesium chloride (1 M)
Reagent Final concentration Quantity or Volume Magnesium chloride hexahydrate 1 M 20.33 g Dissolve the magnesium chloride hexahydrate in Milli-Q water, add the volume up to exactly 100 mL, and store at 4 °C.
HEPES Krebs solution
Reagent Final concentration Quantity or Volume Sodium chloride 134 mM 1.593 g Potassium chloride 6 mM 89.46 mg Glucose 7 mM 252.21 mg HEPES 10 mM 476.6 mg Calcium chloride (1 M) 2 mM 400 μL Magnesium chloride (1 M) 1 mM 200 μL Dissolve the reagents in Milli-Q water, adjust the pH to 7.4, and add the volume up to exactly 200 mL. The solution should be freshly prepared on the day of experiment.
SMDS solution
Reagent Final concentration Quantity or Volume Sodium chloride 60 mM 1.75 g Sodium gluconate 80 mM 8.73 g Potassium chloride 5 mM 186.38 mg Magnesium chloride hexahydrate 2 mM 203.3 mg Glucose 10 mM 900.75 mg HEPES 10 mM 1.19 g Dissolve the reagents in Milli-Q water, adjust the pH to 7.4, and add up to exactly 500 mL. After preparation, distribute the solution into 50 mL tubes and store them at -20 °C. Thaw one 50 mL SMDS solution on the day of the experiment before use.
Wash buffer A and wash buffer B
Prepare PLA wash buffer A and B ahead by dissolving the PLA buffer sachets each in 1 L of Milli-Q. Filter sterilise both buffers using a syringe and a 0.2 μm pore filter and store at 4 °C.
Stock solutions
Reagent Concentration Minimum volume needed BSA 10 mg/mL 200 μL DTT 10 mg/mL 150 μL Papain 5 mg/mL 100 μL Collagenase F 10 mg/mL 70 μL Collagenase H 5 mg/mL 40 μL Tube 1
Stock solution Final concentration Quantity or Volume BSA (10 mg/mL) 1 mg/mL 100 μL DTT (10 mg/mL) 1.5 mg/mL 150 μL Papain (5 mg/mL) 0.5 mg/mL 100 μL SMDS n/a 650 μL Total n/a 1,000 μL Tube 2
Stock solution Final concentration Quantity or Volume BSA (10 mg/mL) 1 mg/mL 100 μL Collagenase F (10 mg/mL) 0.7 mg/mL 70 μL Collagenase H (5 mg/mL) 0.2 mg/mL 40 μL *Calcium Chloride (100 mM) 100 µM 1 μL SMDS n/a 790 μL Total n/a 1000 μL *A stock solution of calcium chloride can be pre-made and stored at room temperature for repeated use.
Laboratory supplies
Surgical scissors (Fine Science Tools, Mayo-Stille Scissors, catalog number: 14101-14)
Dissection forceps (Fine Science Tools, Fine Forceps, catalog number: 11252-00)
Dissection scissors (Fine Science Tools, Spring scissors, catalog number: 15020-15)
Dissection dish (Silicone coated)
Sewing pins
1.5 mL microcentrifuge tube (Eppendorf, catalog number: 0030120086)
50 mL Falcon tube (Falcon)
100/200 mL glass beaker
50 mL syringe (BD Plastipak)
0.2 μm pore syringe filter (Sigma-Aldrich, catalog number: WHA9914)
Disposable Pasteur pipette (Thermo Fisher, catalog number: 202-1SPK)
24-well cell culture plate (Thermo Fisher, catalog number: 144530)
Styrofoam ice box
Fire-polished glass pipette (custom-made, see Figure 1)
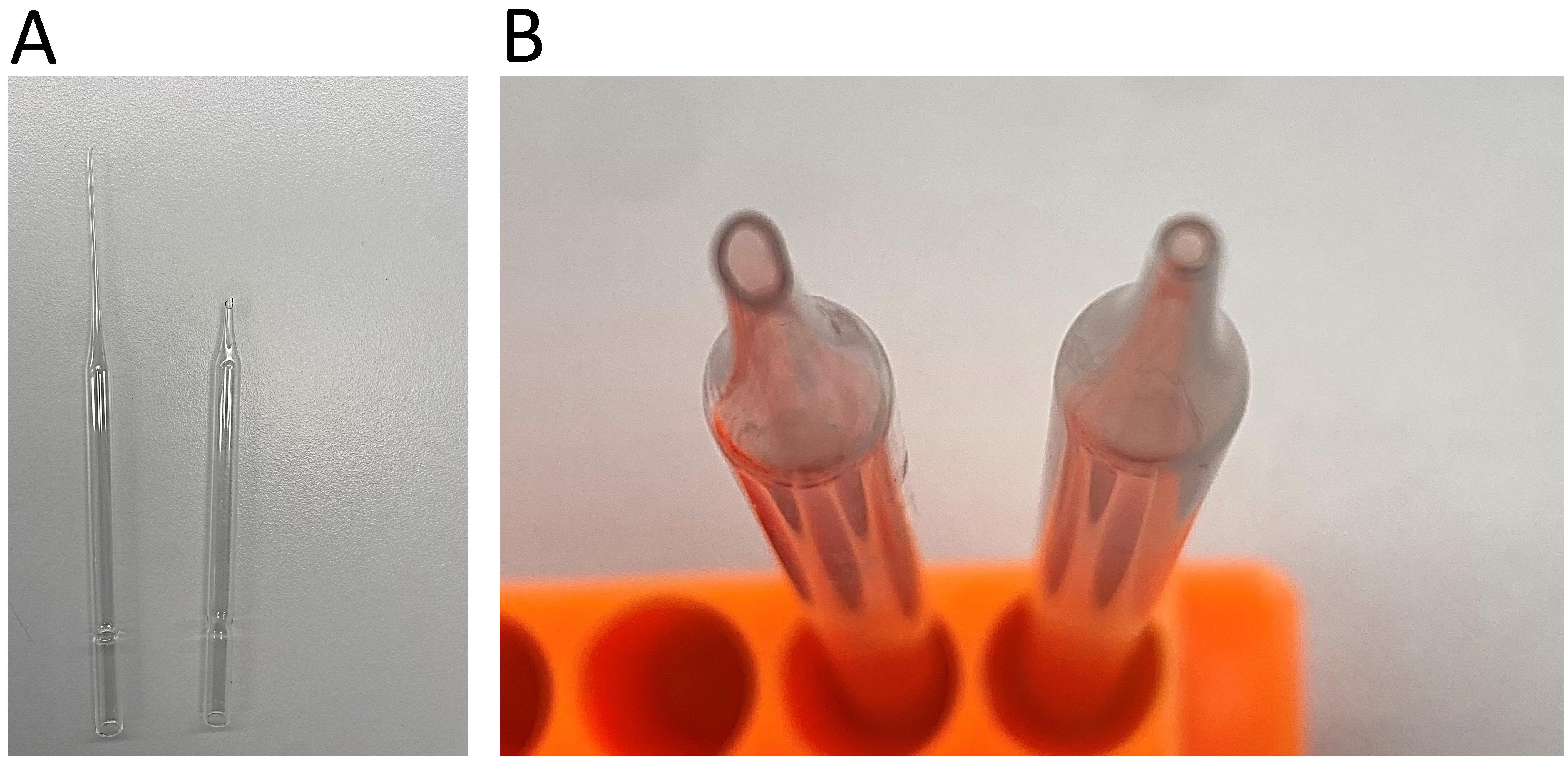
Figure 1. Fire-polishing of the glass Pasteur pipette. A. Remove and discard the long end of the pipette. B. Rotate the end of the pipette in a Bunsen flame until its edges are polished and the opening is smoothed and narrowed. Comparison of the opening before (left) and after (right) fire polishing.Humidified chamber (custom-made)
Cover glass 12-mm circles (VWR, catalog number: 76355-906)
Microscope slides (VWR, catalog number: 16004-422)
Clear nail polish (any)
Equipment
Pipettes (ranging from 1 to 1,000 μL)
Heat block (37 °C)
Bunsen burner
Plate rocker at room temperature
37 °C, 5% CO2 cell culture incubator
Dissecting microscope (Olympus SZX7 Stereomicroscope)
Cell microscope (Leitz Labovert inverted light microscope, 10× objective)
ZEIS LSM780/LSM900 microscope or any other laser scanning confocal microscope
Software
ImageJ (version 1.53k, July 2021)
GraphPad PRISM (version 9, October 2020)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
van der Horst, J. and Jepps, T. A. (2024). Proximity Labelling to Quantify Kv7.4 and Dynein Protein Interaction in Freshly Isolated Rat Vascular Smooth Muscle Cells. Bio-protocol 14(6): e4961. DOI: 10.21769/BioProtoc.4961.
分类
分子生物学 > 蛋白质 > 蛋白质-蛋白质相互作用
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link