- EN - English
- CN - 中文
Mesenchymal Stromal Cell (MSC) Functional Analysis—Macrophage Activation and Polarization Assays
间充质干细胞(MSC)功能分析—巨噬细胞激活与极化测定
发布: 2024年03月20日第14卷第6期 DOI: 10.21769/BioProtoc.4957 浏览次数: 4328
评审: Vivien J. Coulson-ThomasAnonymous reviewer(s)
Abstract
Stem cell–based therapies have evolved to become a key component of regenerative medicine approaches to human pathologies. Exogenous stem cell transplantation takes advantage of the potential of stem cells to self-renew, differentiate, home to sites of injury, and sufficiently evade the immune system to remain viable for the release of anti-inflammatory cytokines, chemokines, and growth factors. Common to many pathologies is the exacerbation of inflammation at the injury site by proinflammatory macrophages. An increasing body of evidence has demonstrated that mesenchymal stromal cells (MSCs) can influence the immunophenotype and function of myeloid lineage cells to promote therapeutic effects. Understanding the degree to which MSCs can modulate the phenotype of macrophages within an inflammatory environment is of interest when considering strategies for targeted cell therapies. There is a critical need for potency assays to elucidate these intercellular interactions in vitro and provide insight into potential mechanisms of action attributable to the immunomodulatory and polarizing capacities of MSCs, as well as other cells with immunomodulatory potential. However, the complexity of the responses, in terms of cell phenotypes and characteristics, timing of these interactions, and the degree to which cell contact is involved, have made the study of these interactions challenging. To provide a research tool to study the direct interactions between MSCs and macrophages, we developed a potency assay that directly co-cultures MSCs with naïve macrophages under proinflammatory conditions. Using this assay, we demonstrated changes in the macrophage secretome and phenotype, which can be used to evaluate the abilities of the cell samples to influence the cell microenvironment. These results suggest the immunomodulatory effects of MSCs on macrophages while revealing key cytokines and phenotypic changes that may inform their efficacy as potential cellular therapies.
Key features
• The protocol uses monocytes differentiated into naïve macrophages, which are loosely adherent, have a relatively homogeneous genetic background, and resemble peripheral blood mononuclear cells–derived macrophages.
• The protocol requires a plate reader and a flow cytometer with the ability to detect six fluorophores.
• The protocol provides a quantitative measurement of co-culture conditions by the addition of a fixed number of freshly thawed or culture-rescued MSCs to macrophages.
• This protocol uses assessment of the secretome and cell harvest to independently verify the nature of the interactions between macrophages and MSCs.
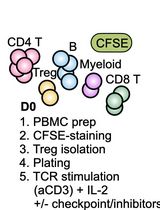
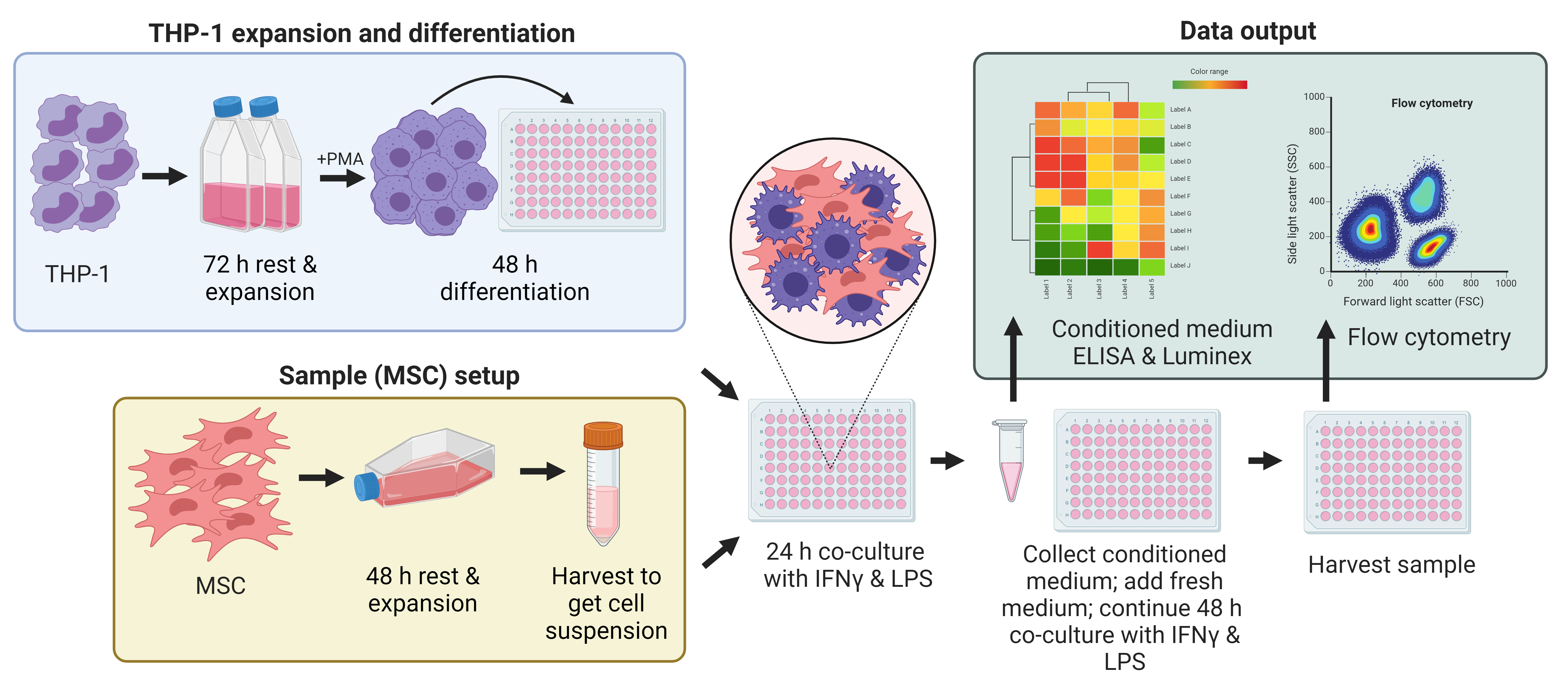
Graphical overview
Background
Mesenchymal stromal cell (MSC) transplants, through their immunomodulatory and regenerative properties, have emerged as a viable therapeutic approach in a wide range of diseases. MSCs isolated from bone marrow, umbilical cord, and adipose tissue have been used in clinical trials to treat conditions as far-reaching as traumatic brain injury, autism spectrum disorder, osteoarthritis, immune system disorders, and cardiovascular diseases [1]. To standardize such treatments and attempt to ensure successful outcomes, various approaches have been suggested to characterize the cells in functional or potency assays in vitro, to better predict treatment responses, e.g., DOSES [donor; origin; separation method; exhibited characteristics (cell behavior); and site of delivery] [2]. A known property of MSCs is their ability to modulate the phenotype of macrophages.
Monocyte-derived macrophages are important cells in the inflammatory cascade, key to tissue healing and regeneration. In the early stages of tissue injury or throughout chronic inflammatory pathologies, macrophages are driven to polarize to an M1 state, referred to as classically activated, and will secrete a range of proinflammatory cytokines and chemokines such as tumor necrosis factor-alpha (TNFα), interleukin-12 (IL-12), and CC-chemokine receptor-7 (CCR7), and express major histocompatibility complex (MHC) II cell surface receptor (HLA-DR) [3]. As the inflammation subsides, the predominant macrophage phenotype shifts to M2 (alternative activation) and is associated with the release of anti-inflammatory cytokines such as interleukin-10 (IL-10), interleukin-13 (IL-13), and interleukin-1 receptor antagonist (IL-1RA) [4]. Polarization to M2 reparatory macrophages, effected by MSCs in situ, can result in the restoration of homeostasis and tissue repair as well as the reduction of inflammation [5].
MSCs in culture are responsive to culture conditions and secrete both free and exosome-encapsulated cytokines and microRNA. To establish the cell culture techniques that best prepare MSCs for in vivo delivery and subsequent immunomodulation of macrophage phenotype and function in the body, in vitro co-culture assays can be used to study the crosstalk between these cell types to recapitulate the complex interactions in vivo. However, studies with macrophage cultures can be challenging due to the difficulties in attaining a pure population of macrophages and in the harvesting of these cells, as they strongly adhere to tissue culture surfaces when differentiated/activated. The Tohoku Hospital Pediatrics-1 (THP-1) cell line has been widely used to study monocyte and macrophage function in vitro, in the presence of a wide range of other cell types such as intestinal cells, adipocytes, T-lymphocytes, platelets, and vascular smooth muscle cells [6].
The proposed mechanisms of action of MSCs [7–13] center on the abatement of the inflammatory condition by the reduction in a number of proinflammatory cytokines such as TNFα, IL-6, IL-8, and IL1-β. In the first part of this assay, the ability of delivered MSCs to counter this proinflammatory condition is measured by the suppression of the release of TNFα and other cytokines from M1-like macrophages. In the clinic, the mobilization of MSCs to counter inflammation, as seen with oxygen therapies administered to patients with respiratory and cognitive disorders, may mimic a similar endogenous response by MSCs [14,15].
MSC administration generates concomitant decreases in proinflammatory cytokine release and increases in anti-inflammatory mediator production by macrophages [16,17]. These changes are accompanied by the polarization of M1 towards M2 macrophages [18]. This shift is examined in the second part of the assay.
In consideration of the role of cell therapy and, in particular, the delivery of allogeneic MSCs in ameliorating disease conditions, the study of the interactions of MSCs and macrophages is pertinent. Herein, we have established an in vitro assay system to study both macrophage activation and polarization in the presence of delivered MSCs. Freshly thawed and culture-rescued cells can differ in their ability to activate and polarize macrophages. Freshly thawed cells have disturbed membrane physiology and display a differential release of paracrine mediators and microparticles (exosomes, microvesicles, and apoptotic bodies) compared with cultured cells. Although freshly thawed cells are less likely to persist in the body due to innate immune cascades, coagulation response, etc., their passive release of microparticles is likely augmented compared with culture-rescued cells. However, stronger immunomodulatory capacity has been attributed to freshly cultured cells [19]. These assays establish key parameters for studying the interaction of MSCs and macrophages in vitro.
Materials and reagents
Biological materials
THP-1 cell vial, which has been stored in liquid nitrogen (LN2) at vapor phase (ATCC, catalog number: TIB-202)
Human MSCs derived from bone marrow or umbilical cord tissue, which have been stored frozen in a cryovial and maintained in LN2 at vapor phase (RoosterBio Inc., Frederick, MD, catalog number: RoosterVial-hBM or supplied by Duke University MC3)
Reagents
RPMI 1640 containing L-glutamine and 10 mM HEPES (ATCC, catalog number: 30-2001)
RoosterNourishTM -MSC (RoosterBio Inc, catalog number: KT-001)
Prime-XV MSC Expansion XSFM (Irvine Scientific, catalog number: 91149)
Penicillin/Streptomycin (P/S) (Millipore Sigma, catalog number: P4333)
Fetal bovine serum (FBS), heat inactivated (H.I.) and characterized (Cytiva, catalog number: SH30071.01)
β-mercaptoethanol (BME) (Thermo Fisher Scientific, catalog number: 21985023)
Dimethylsulfoxide (DMSO) (Sigma-Aldrich, catalog number: C6164)
Phorbol 12-myristate 13 acetate (PMA) (Sigma-Aldrich, catalog number: P1585)
PLASMA-LYTE A (Baxter Intl Inc., catalog number: 2B2543Q)
Human serum albumin (HSA) (Albutein) 25% (Grifols, catalog number: 68516-5216-02)
Phosphate buffered saline (PBS) Ca2+/Mg2+ free, low endotoxin (Millipore Sigma, catalog number: TMS-012-A)
Interferon-gamma (IFN-γ) (Thermo Fisher Scientific, catalog number: 300-02)
Lipopolysaccharide (LPS) (Millipore Sigma, catalog number: L4391)
Sterile endotoxin-free water (VWR, catalog number: 75799-280)
TrypLE Express 1× (Millipore Sigma, catalog number: 12605028)
Zombie UV Fixable Viability kit (BioLegend, catalog number: 423107)
Human TruStain FcXTM (BioLegend, catalog number: 422302)
Antibodies (BioLegend, see Table 1)
Table 1. Antibody information for cell phenotype panel
Surface marker Final dilution Conjugate Vendor Catalog Clone Human macrophage
/MSC panel antibodiesHLA-DR 1:20 (see General note 6) FITC BioLegend 307620 L243 CD163 1:20 PE BioLegend 333606 GHI/61 CD73 1:20 PerCP/Cy5.5 BioLegend 344014 AD2 CD206 1:20 APC BioLegend 321110 15-2 CD14 1:20 PE/Cy7 BioLegend 367112 63D3 Zombie UV 1:1,000 - BioLegend 423107 - Compensation beads Anti-Mouse Ig +/- control beads One drop/500 µL FC buffer - BD 552843 - ArC reactive beads for use with Zombie UV One drop/500 µL FC buffer - Thermo Fisher A10346 - Anti-mouse negative and positive control beads (Thermo Fisher Scientific, catalog number: 552843)
ArC reactive beads (Thermo Fisher Scientific, catalog numbers: A10346 and A10628)
Cytofix fixation buffer (BD Biosciences; catalog number: 554655)
ELISA kit TNFα (e.g., Thermo Fisher Scientific, catalog number: PO1375)
Luminex Cytokine Kit Magnetic 30 Plex Panel (Thermo Scientific, catalog number: LHC6003M)
Solutions
THP-1 expansion medium (see Recipes)
THP-1 differentiation medium (see Recipes)
Diluting buffer (see Recipes)
PMA diluted (see Recipes)
LPS stock (see Recipes)
IFN-γ stock (see Recipes)
Co-culture medium (see Recipes)
Zombie-UV (see Recipes)
Blocking buffer (see Recipes)
FC buffer (see Recipes)
Antibody cocktail (see Recipes)
Recipes
THP-1 expansion medium
Reagent Final concentration Volume RPMI 1640 79% 394.5 mL FBS H.I. 20% 100 mL P/S 1% 5 mL BME 0.1% 0.5 mL Total 100% 500 mL THP-1 differentiation medium
Reagent Final concentration Volume RPMI 1640 98% 48.5 mL FBS H.I. 1% 0.5 mL P/S 1% 0.5 mL PMA diluted 100 ng/mL 0.5 mL Total 100% 50 mL Diluting buffer
Reagent Final concentration Volume PLASMA-LYTE A 96% 48 mL HSA (25%) 4% (1%) 2 mL Total 100% 50 mL PMA diluted
Make a stock solution of 5 mg of PMA in 1 mL of DMSO. Then, add 5 µL of stock to 2.5 mL of RPMI 1640 (no additives). Filter sterilize through a 0.2 µm filter and then add to 1% FBS media as above.
Reagent Final concentration Volume PMA stock (5 mg/mL in DMSO) 10 µg/mL 5 µL Medium RPMI 1640 2.5 mL Total 100% 2.5 mL LPS stock
Reagent Final concentration Quantity LPS 1 mg/mL 1 mg Sterile water 1 mL Total 100% 1 mL IFN-γ stock
Reagent Final concentration Quantity IFN-γ 100 µg/mL 100 µg Sterile water 1 mL Total 100% 1 mL Co-culture medium
Reagent Final concentration Volume RPMI 1640 98% 49 mL FBS H.I. 1% 0.5 mL P/S 1% 0.5 mL LPS 100 ng/mL 5 µL IFN-γ 100 ng/mL 50 µL Total 100% 50 mL Zombie- UV
Reagent Final concentration Volume Zombie-UV (reconstituted) 0.1% 5 µL PBS Ca2+/Mg2+ free (1×) 99.9% 5 mL Total 100% 5 mL Blocking buffer
Reagent Final concentration Volume TruStain FcX 10% 1 mL FC buffer 90% 9 mL Total 100% 10 mL FC buffer
Reagent Final concentration Volume FBS H.I. 2% 2 mL PBS Ca2+/Mg2+ free (1×) 98% 98 mL Total 100% 100 mL Antibody cocktail (70 wells)
Reagent Final concentration Volume Antibodies (350 µL each) 50% 1.75 mL FC buffer 50% 1.75 mL Total 100% 3.5 mL
Laboratory supplies
Non-treated T-25, T-75, and T-175 suspension flasks (e.g., Corning, ultralow attachment, catalog number: 4616, 3814)
Tissue culture treated T-225 flasks (e.g., Corning, CELLBIND, catalog number: 3293)
Cryogenic vials for vapor phase (e.g., Corning, Fisher Scientific, catalog number: 431386)
Freezing container (e.g., Corning, Millipore Sigma, model: CoolCell LX, catalog number: CLS432002)
Serological pipettes [e.g., VWR, catalog numbers: 414004-266 (5 mL); 414004-267 (10 mL); 414004-268 (25 mL)]
Micropipette tips, P10P1000 µL [e.g., Fisher Scientific, Biotix uTIP filter, catalog numbers: 12-111-000 (10 µL); 12-111-004 (300 µL); 12-111-132 (1,000 µL)]
Via-1-CassetteTM cartridges (Chemometec, catalog number: 941-0012)
CELLBIND 96-well cell culture plate (Corning, Millipore Sigma, catalog number: CLS3300)
96-well culture plate for media storage (e.g., Corning, tissue culture treated, catalog number: 3894)
V-bottom 96-well plate (Corning, Millipore Sigma, catalog number: CLS3894)
Parafilm (e.g., Sigma-Aldrich, catalog number: P7793)
Pre-saturated alcohol wipes (e.g., VWR, catalog number: 33503-136)
Equipment
Biosafety cabinet (BSC) level A2 (e.g., Thermo Fisher Scientific, catalog number: 1377)
Incubator set to 5% CO2 and 37 °C (e.g., Thermo Fisher Scientific, model: HeraCell VIOS)
Cell counter, (e.g., Chemometec, model: NucleoCounter NC-200TM)
Refrigerated centrifuge capable of reaching 500× g and using 50 mL conical tubes and microplates (e.g., Thermo Fisher Scientific, model: Megafuge 8R, catalog number: 75007214)
Micropipettes P10–1,000 µL (e.g., Eppendorf, Research Plus range, catalog number: 312300020)
Pipette controller (e.g., Eppendorf, model: Easypet, catalog number: 2231000955)
Inverted microscope (e.g., Thermo Fisher Scientific, model EVOS M5000, catalog number: AMF5000)
Microplate absorbance reader (e.g., Cytation 5)
Flow cytometer, e.g., CytoFlex (Beckman Coulter), LSRFortessa (BD)
Luminescence microplate reader (e.g., Bio-Rad, model: Bioplex 200)
Software and datasets
Flow cytometry software is needed for data analysis. This can be either FlowJoTM (BD Biosciences) or CytoBank (Beckman Coulter) software
Prism 9 (GraphPad, San Diego, CA)
JMP statistical software (Pro 17)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Stevens, H. Y., Jimenez, A. C., Wang, B., Li, Y., Selvam, S. and Bowles-Welch, A. C. (2024). Mesenchymal Stromal Cell (MSC) Functional Analysis—Macrophage Activation and Polarization Assays. Bio-protocol 14(6): e4957. DOI: 10.21769/BioProtoc.4957.
分类
免疫学 > 免疫机理 > 体外模型
细胞生物学 > 基于细胞的分析方法 > 炎症反应
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link