- EN - English
- CN - 中文
Simple Rescue of Opaque Tissue Previously Cleared by iDISCO
通过iDISCO简单恢复先前清除的不透明组织
(*contributed equally to this work) 发布: 2024年03月05日第14卷第5期 DOI: 10.21769/BioProtoc.4948 浏览次数: 2210
评审: Hong LianOneil Girish BhalalaAnonymous reviewer(s)
Abstract
Recent advancements in tissue-clearing techniques and volumetric imaging have greatly facilitated visualization and quantification of biomolecules, organelles, and cells in intact organs or even entire organisms. Generally, there are two types of clearing methods: hydrophobic and hydrophilic (i.e., clearing with organic or aqueous solvents, respectively). The popular iDISCO approach and its modifications are hydrophobic methods that involve dehydration, delipidation, decolorization (optional), decalcification (optional), and refractive-index (RI) matching steps. Cleared samples are often stored for a relatively long period of time and imaged repeatedly. However, cleared tissues can become opaque over time, which prevents accurate reimaging. We reasoned that the resurgent haziness is likely due to rehydration, residual lipids, and uneven RI deep inside those tissue samples. For rescue, we have developed a simple procedure based on iDISCO. Beginning with a methanol dehydration, samples are delipidated using dichloromethane, followed by RI matching with dibenzyl ether (DBE). This simple method effectively re-clears mouse brains that have turned opaque during months of storage, allowing the user to effectively image immunolabeled samples over longer periods of time.
Key features
• This simple protocol rescues previously cleared tissue that has turned opaque.
• The method does not cause detectable loss of immunofluorescence from previously stained samples.
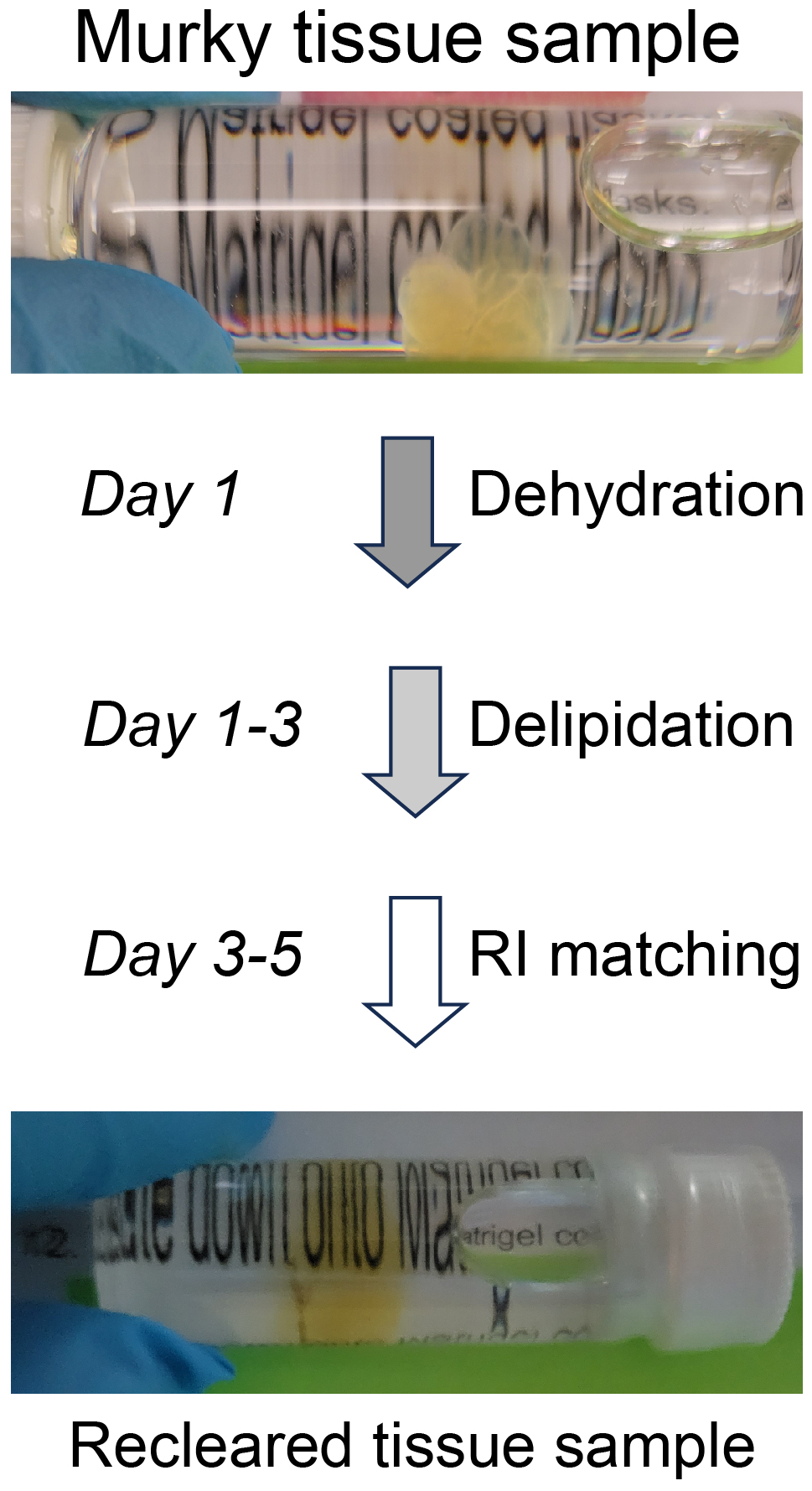
Graphical overview
Background
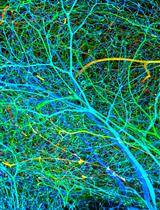
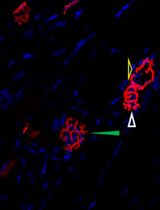
Tissue clearing paired with light-sheet microscopy rejuvenated the century-old histochemistry field [1]. Clearing can render organs or even whole animals transparent [2], while the light-sheet microscopy can image fluorescent labels deep inside tissues or bodies [3]. Available clearing techniques generally fall into two categories based on the solvents used, specifically whether using hydrophilic or hydrophobic (including hydrogel-based methods) solutions [4]. Despite technical differences, the underlying principles between the two are the same, i.e., substituting molecules that diffract lights (e.g., lipids, pigments, and calcium phosphate) with other molecules that match the refractive index (RI) of the imaging media [2]. The transparency of cleared tissues allows fluorescence imaging of biomolecules deep inside the tissue (i.e., intrinsically expressed fluorescent proteins or after labeling with fluorescently tagged antibodies), which is further facilitated by light-sheet microscopy that illuminates the sample using an adjustable thin sheet of UV light [3]. Notably, fluorophore-tagged antibodies are often used to boost the detection of endogenous fluorescent proteins. iDISCO, a hydrophobic clearing method integrated with immunohistochemistry [5], has become very popular and given rise to multiple derivatives including uDISCO [6] and vDISCO [7]. Conventionally, cleared samples are stored in RI-matching media, often for months, for repeated imaging (please see https://idisco.info for more information). However, during storage, iDISCO-cleared specimens can turn cloudy and some even become opaque, which makes them unsuitable for imaging accurately by light-sheet microscopy. We suspected that such changes in tissue opacity with storage arose from residual lipids, a loss of RI matching deep inside the tissue, or the rehydration of samples by moisture in the air or dissolved in the storage solvent. To counteract those changes, we developed a simple rescue protocol based on immunofluorescence-compatible iDISCO. The approach is comprised by three steps—dehydration, delipidation, and RI rematching. The protocol we describe can successfully re-clear entire, murky mouse brains that had been previously cleared by iDISCO. We imaged the re-cleared brains by light-sheet microscopy, observing little or no loss of immunofluorescence as compared to imaging prior to storage, allowing the samples to be stored and studied far beyond the time of initial scanning.
Materials and reagents
Biological materials
Murky tissues previously cleared by iDISCO and possibly other hydrophobic clearing methods
Methanol (Sigma-Aldrich, catalog number: 179337)
Dichloromethane (Sigma-Aldrich, catalog number: 270997)
Dibenzyl ether (Sigma-Aldrich, catalog number: 33630)
Dichloromethane and methanol mix (see Recipes)
Recipes
Dichloromethane and methanol mix (per sample)
Reagent Final concentration (vol%) Volume Dichloromethane 66% 3.3 mL Methanol 34% 1.7 mL Total n/a 5.0 mL The mix should be made freshly and kept at room temperature before use.
Laboratory supplies
Organ holding forceps (e.g., Fine Science Tools, catalog number: 11031-15)
Screw-cap glass vials (e.g., Millipore Sigma, catalog number: 27151)
Nitrile gloves (any brand)
Kimwipes and paper towels (any brand)
Equipment
Fume hood (e.g., Hamilton Lab, model: SafeAire II)
Orbital shaker (e.g., Stovall Life Science, Belly Dancer, model: BDRAA115S)
Light-sheet microscope (e.g., Miltenyi Biotec, model: UltraMicroscope II Blaze)
Computer workstation equipped with, at least, Intel Xeon CPU, 128 G RAM, GPU with 16 GB memory, 2 TB storage, and USB3.0 port.
Software and datasets
Cloud-based lab management software (e.g., Labguru.com) is recommended to track and record the rescue procedure
Microscope system control and image acquisition software (e.g., ImSpector 7.5.3, free)
Image processing software (e.g., ImageJ1.53, free)
Atlas-matching and immunofluorescence quantification software (e.g., NeuroInfo, licensed by MBF Bioscience)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Mesa, H., Meade, J., Gajewski-Kurdziel, P., Blakely, R. D. and Zhang, Q. (2024). Simple Rescue of Opaque Tissue Previously Cleared by iDISCO. Bio-protocol 14(5): e4948. DOI: 10.21769/BioProtoc.4948.
分类
神经科学 > 神经解剖学和神经环路 > 荧光成像
细胞生物学 > 细胞成像 > 固定组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link