- EN - English
- CN - 中文
A Versatile Pipeline for High-fidelity Imaging and Analysis of Vascular Networks Across the Body
用于全身血管网络高保真成像和分析的多功能管道
发布: 2024年02月20日第14卷第4期 DOI: 10.21769/BioProtoc.4938 浏览次数: 2995
评审: Pilar Villacampa AlcubierreXiaokang Wu
Abstract
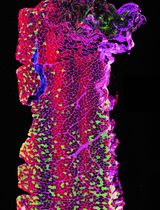
Structural and functional changes in vascular networks play a vital role during development, causing or contributing to the pathophysiology of injury and disease. Current methods to trace and image the vasculature in laboratory settings have proven inconsistent, inaccurate, and labor intensive, lacking the inherent three-dimensional structure of vasculature. Here, we provide a robust and highly reproducible method to image and quantify changes in vascular networks down to the capillary level. The method combines vasculature tracing, tissue clearing, and three-dimensional imaging techniques with vessel segmentation using AI-based convolutional reconstruction to rapidly process large, unsectioned tissue specimens throughout the body with high fidelity. The practicality and scalability of our protocol offer application across various fields of biomedical sciences. Obviating the need for sectioning of samples, this method will expedite qualitative and quantitative analyses of vascular networks. Preparation of the fluorescent gel perfusate takes < 30 min per study. Transcardiac perfusion and vasculature tracing takes approximately 20 min, while dissection of tissue samples ranges from 5 to 15 min depending on the tissue of interest. The tissue clearing protocol takes approximately 24–48 h per whole-tissue sample. Lastly, three-dimensional imaging and analysis can be completed in one day. The entire procedure can be carried out by a competent graduate student or experienced technician.
Key features
• This robust and highly reproducible method allows users to image and quantify changes in vascular networks down to the capillary level.
• Three-dimensional imaging techniques with vessel segmentation enable rapid processing of large, unsectioned tissue specimens throughout the body.
• It takes approximately 2–3 days for sample preparation, three-dimensional imaging, and analysis.
• The user-friendly pipeline can be completed by experienced and non-experienced users.
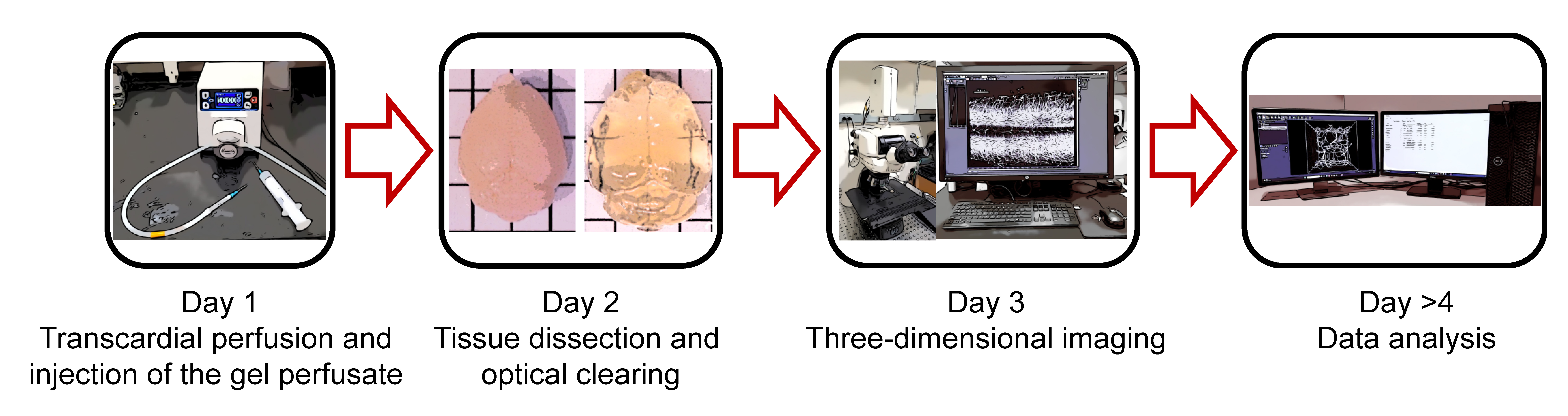
Graphical overview
Background
Vasculature plays a key role during the growth, maintenance, and repair of tissues throughout the body. Accumulating evidence suggests that the slow and protracted reorganization of vascular networks can promote recovery of function (Felmeden et al., 2003; Evans et al., 2021) but may also contribute to neuropathological hallmarks of injury and disease (Ostergaard et al., 2016).
A critical point to consider is that standard methods to study vasculature changes in laboratory settings have proven inaccurate and labor-intensive, lacking the inherent three-dimensional structure of the vasculature. Conventionally, visualization of blood vessels in tissues of interest often employs immunohistochemical (IHC) techniques. This approach uses thin tissue sections to detect endothelial cells through vessel wall stains. The resulting two-dimensional images do not capture the inherent three-dimensional, tortuous structure of vasculature networks (Rust et al., 2020). Furthermore, IHC is a multistep process, increasing the likelihood of introducing error, non-experimental variables (e.g., lot-to-lot variability that contributes to inconsistent results in assays using antibodies), or experimenter bias to analysis, as only a small fraction of the tissue is typically processed. Thus, quantifying the vascular response to injury or disease is often limited to the mean fluorescent intensity of IHC stains, which is highly dependent on technical consistency and subject to bias (Marien et al., 2016; Cheung et al., 2020). Methods of three-dimensional imaging of vasculature networks are currently available. However, they are niche in their applications throughout the body and are largely constrained by expensive or otherwise inaccessible imaging equipment and methodologies including tomography, magnetic resonance imaging, and photoacoustic imaging (Xiong et al., 2017; Pac et al., 2022; Menozzi et al., 2023).
To overcome these barriers, we have developed a practical and accessible pipeline that permits robust and highly reproducible qualitative and quantitative three-dimensional analysis of vascular networks that can resolve changes to the capillary level. Initially, we detail our approach for vasculature tracing and preparing unsectioned tissue samples throughout the body. Then, we present a methodology for optically clearing neuronal and non-neuronal tissues (Susaki et al., 2015) and provide the necessary steps to acquire three-dimensional images using conventional laser scanning confocal microscopy. Lastly, we detail our pipeline for vessel segmentation using AI-based convolutional reconstruction (Imaris software, paid subscription) or semi-automated three-dimensional reconstruction (ImageJ software, freely available). This framework can consistently capture subtle changes in capillary structure such as total length, branching points, branch length, and diameter of vessels, providing a robust and user-friendly approach to study vascular networks in health and disease. Additionally, this scalable method can be adapted for volumetric imaging and analysis of vasculature structures in a variety of preclinical models, thus accelerating translation of promising findings to large animals and non-human primates.
Materials and reagents
Biological materials
Adult (7–8-week-old) female and male C57BL/6J mice (The Jackson Laboratory, stock number: 000664)
Reagents
Paraformaldehyde (PFA) (Sigma, catalog number: 441244)
Ketamine hydrochloride (100 mg/mL) (Covertrus, catalog number: 071069)
Xylazine hydrochloride (100 mg/mL) (Rompun)
Albumin-tetramethylrhodamine isothiocyanate bovine (albumin-TRITC) (Sigma, catalog number: A2289)
0.9% Isotonic saline solution (B. Braun, catalog number: R5200)
Gelatin from porcine skin (Sigma, catalog number: G1890)
Phosphate buffered saline (PBS) (Gibco, catalog number: 21600-044)
Urea (Bio-Rad, catalog number: 161-0731)
Quadrol [N,N,N’,N’-Tetrakis(2-hydroxypropyl)ethylenediamine] (Sigma, catalog number: 122262)
Triton-X 100 (Sigma, catalog number: T9284)
Solutions
4% PFA (see Recipes)
80 wt% Quadrol (see Recipes)
Clearing solution (see Recipes)
Gel perfusate (see Recipes)
Ketamine/Xylazine mixture (see Recipes)
Recipes
4% PFA
Reagent Final Concentration Quantity PFA 4% 40 g PBS 1× 1,000 mL Total 4% 1,000 mL 80 wt% Quadrol
Reagent Final Concentration Quantity Quadrol 80 wt% 500 g dH2O n/a 125 g Total 80 wt% 625 g Clearing solution
Reagent Final Concentration Quantity 80 wt% Quadrol 80% 156 g Urea n/a 125 g dH2O n/a 144 g Triton-X 100 n/a 75 g Total 80% 500 g Gel perfusate
Reagent Final Concentration Quantity Gelatin from porcine skin 2% 0.1 g/5 mL Albumin-TRITC 0.05% 2.5 mg/5 mL PBS 1× 5 mL/mouse Total n/a n/a Ketamine/Xylazine anesthetic
Reagent Final Concentration Quantity Ketamine 150 mg/kg body weight n/a Xylazine hydrochloride 15 mg/kg body weight n/a Final n/a
Laboratory supplies
Aluminum foil (Fisher Scientific, Fisherbrand, catalog number: 01-213-100)
Microruler (Fine Science Tools, catalog number: 30086-30)
Fluted filter paper circles (Fisher Scientific, Fisherbrand, catalog number: 09-790-14D)
2 mL Eppendorf tube (Eppendorf, catalog number: 022363352)
50 mL conical centrifuge tube (VWR, catalog number: 89039-656)
100 mL glass beaker (Pyrex, Corning, catalog number: 1000-100)
Glass coverslip (Fisher Scientific, catalog number: 1254588)
Microscope glass slides (Fisher Scientific, Fisherbrand, catalog number: 12-550-15)
23 G needle (BD Biosciences, catalog number: 305193)
10 mL syringe (Henry Schein, catalog number: 570-2620)
1 L round media storage bottle (Pyrex, catalog number: GL45)
Equipment
Vacuum desiccator (Thermo Scientific, catalog number: 08-642-5)
Peristaltic pump (Cole-Parmer, Masterflex, catalog number: 07525)
Vortex mixer (VWR, catalog number: BV1000-GM)
Hotplate stirrer (Benchmark Scientific, catalog number: H400-HS)
Laser scanning microscope (Nikon, model: C2 plus)
Dissection microscope (Zeiss, model: Stemi 2000, catalog number: SP-STEMI2000-TS2)
Computer with 3.3 GHz CPU (Intel or AMD) 6–8 cores, 32–64 GB of RAM, NVIDA Quadro RTX 4000 (8 GB), and multiple fast hard disks or (SATA) SSDs (recommended system when using Imaris software)
Software and datasets
Imaris Microscopy Image Analysis software (Bitplane, Oxford Instruments, v10.0, RRID: SCR_007370) https://imaris.oxinst.com/products/imaris-for-neuroscientists
Free alternative software: FIJI/ImageJ (NIH, RRID: SCR_002285) https://imagej.nih.gov/ij/download.html)
Excel (Microsoft, https://www.microsoft.com/en-us/microsoft-365/p/excel/cfq7ttc0hr4r?activetab=pivot:overviewtab)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Vidman, S., Dion, E. and Tedeschi, A. (2024). A Versatile Pipeline for High-fidelity Imaging and Analysis of Vascular Networks Across the Body. Bio-protocol 14(4): e4938. DOI: 10.21769/BioProtoc.4938.
分类
神经科学 > 神经解剖学和神经环路 > 荧光成像
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link