- EN - English
- CN - 中文
A Simple Immunofluorescence Method to Characterize Neurodegeneration and Tyrosine Hydroxylase Reduction in Whole Brain of a Drosophila Model of Parkinson’s Disease
一种简单的免疫荧光法来表征帕金森病果蝇模型全脑神经变性和酪氨酸羟化酶减少
(*contributed equally to this work) 发布: 2024年02月20日第14卷第4期 DOI: 10.21769/BioProtoc.4937 浏览次数: 1873
评审: Alessandro DidonnaSara BagnoliAnonymous reviewer(s)

相关实验方案
基于 rAAV-α-Syn 与 α-Syn 预成纤维共同构建的帕金森病一体化小鼠模型
Santhosh Kumar Subramanya [...] Poonam Thakur
2025年12月05日 1626 阅读
Abstract
Dopaminergic (DAergic) neurodegeneration in the substantia nigra pars compacta of the human brain is the pathological feature associated with Parkinson’s disease (PD). Drosophila also exhibits mobility defects and diminished levels of brain dopamine on exposure to neurotoxicants mimicking PD. Our laboratory demonstrated in a Drosophila model of sporadic PD that there is no decrease in DAergic neuronal number; instead, there is a significant reduction in tyrosine hydroxylase (TH) fluorescence intensity (FI). Here, we present a sensitive assay based on the quantification of FI of the secondary antibody (ab). As the FI is directly proportional to the amount of TH synthesis, its reduction under PD conditions denotes the decrease in the TH synthesis, suggesting DAergic neuronal dysfunction. Therefore, FI quantification is a refined and sensitive method to understand the early stages of DAergic neurodegeneration. FI quantification is performed using the ZEN 2012 SP2 single-user software; a license must be acquired to utilize the imaging system to interactively control image acquisition, image processing, and analysis. This method will be of good use to biologists, as it can also be used with little modification to characterize the extent of degeneration and changes in the level of degeneration in response to drugs in different cell types. Unlike the expensive and cumbersome confocal microscopy, the present method will be an affordable option for fund-constrained neurobiology laboratories.
Key features
• Allows characterizing the incipient DAergic and other catecholaminergic neurodegeneration, even in the absence of loss of neuronal cell body.
• Great alternative for the fund-constrained neurobiology laboratories in developing countries to utilize this method in different cell types and their response to drugs/nutraceuticals.
Graphical overview
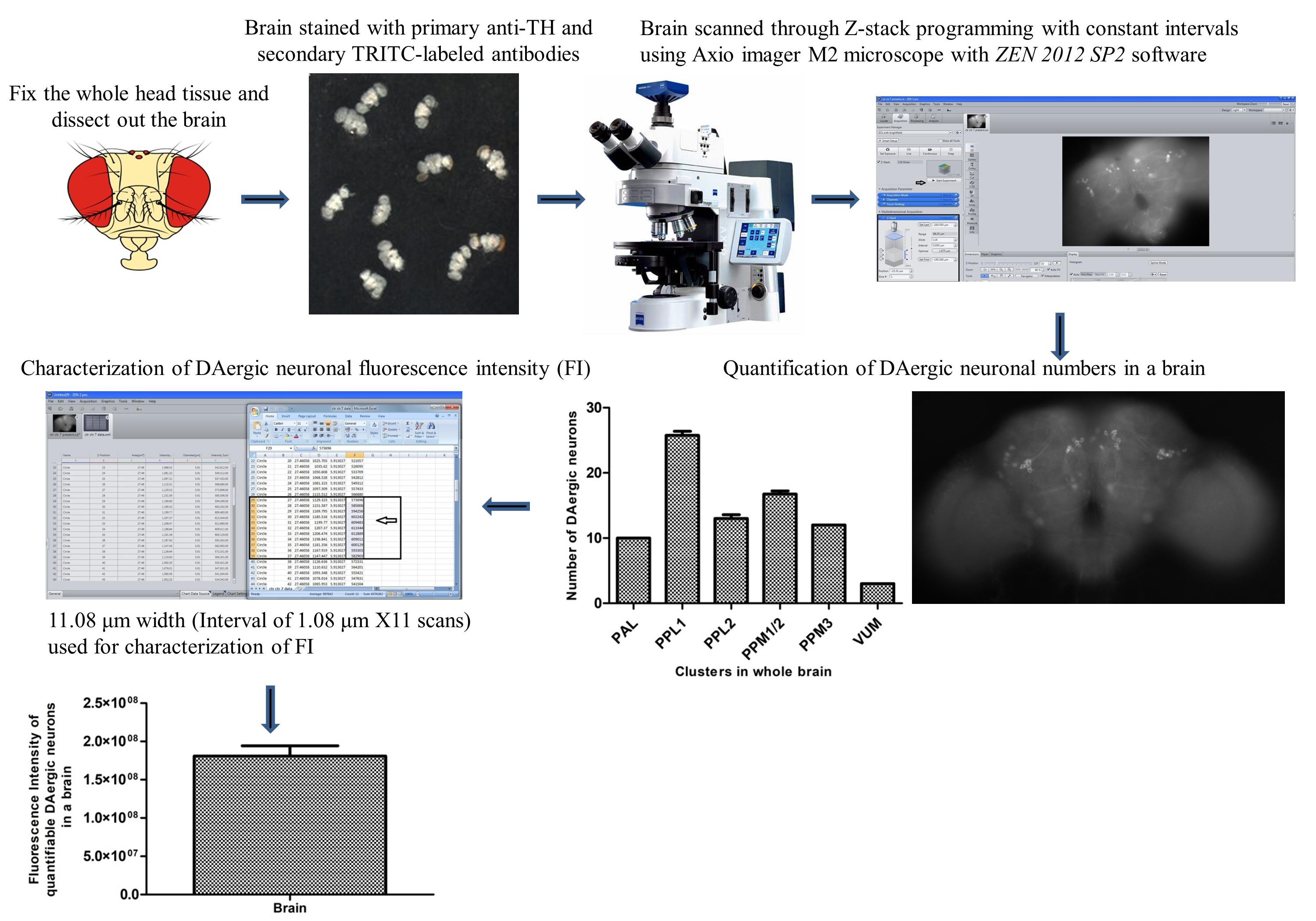
Background
Degeneration of dopaminergic (DAergic) neurons in the substantia nigra pars compacta and noradrenergic neurons in the locus coeruleus region of the human brain is the characteristic pathological feature of Parkinson’s disease (PD) subjects. Tyrosine hydroxylase (TH) is the marker protein for DAergic neurons, as it is the rate-limiting enzyme in the synthesis of dopamine. Hence, demonstration of the DAergic degeneration phenotype using TH immunostaining is critical in the development and validation of animal models of PD. For the first time, in an α-synuclein Drosophila model of PD, Feany and Bender [1] demonstrated a progressive, age-dependent locomotor dysfunction similar to the PD subjects accompanied by a loss of DAergic neurons. Since then, many labs have been using the fly to model PD [2–13].
One prime feature of Drosophila PD models is the DAergic cell loss, as claimed by several independent labs [1,2,6,12,14–19]; however, some other labs found no loss in neuronal numbers [7,9,20–23]. Further neurotoxins such as paraquat and rotenone have been used to develop the Drosophila model of PD. In most cases, cluster-specific loss of DAergic neurons [6,24–27] or no variation in the number of DAergic neurons [3,7,13,21,22,28] were observed. TH immunostaining and green fluorescent protein (GFP) reporter-based methods have typically been adopted to quantify DAergic neurons in the whole Drosophila brain. The reduction in fluorescence intensity (FI) of TH immunostaining or GFP signal has been termed neuronal dysfunction [7].
Here, we describe a sensitive fluorescence microscopy–based assay developed in our laboratory [28], less expensive and more user friendly than cumbersome confocal microscopy, to characterize DAergic neuronal dysfunction even in the absence of loss of neuronal cell bodies. Through this assay, it is feasible to characterize the early DAergic neurodegeneration, which will be of great support in following the progression of the disease; the same can be employed to understand the neuroprotective efficacy of small molecules/nutraceuticals/drugs. With appropriate modifications, this method can also be used for identifying other cell type–specific neurodegeneration in fly models of different neurodegenerative disease(s).
Materials and reagents
Drosophila melanogaster [Oregon K (OK) flies, procured from the National Drosophila Stock Center, Mysore University, Mysuru, India, were raised in food media containing sucrose, yeast, agar-agar, and propionic acid, and maintained in a fly environment chamber and used in the current study [3,10,13,28]]. Depending on the nature of the experiment, flies with and/or without neurotoxicant treatment (sporadic PD model) and transgenic fly model(s) can be employed.
Sterilized 1.5 mL centrifuge tubes (Tarsons, catalog number: 500010)
ParafilmTM wrapping film (Bemis, catalog number: PM996)
Conical flask (Borosil, catalog number: 5100)
Magnetic stirrer bar #8 mm × 40 mm (Tarsons, catalog number: 4113)
SPINNOTTM digital magnetic stirrer hotplate (Tarsons, catalog number: 6090)
Sterilized micro tips (Tarsons, catalog number: 521010)
Freshwrapp aluminum foil 9–11 μm (Hindalco, catalog number: HV2241)
Glass plate (Suwimut, catalog number: B08FRB2NTM)
Fingernail polish (FacesCanada, catalog number: CC4403)
Glass spacer (Borosil, catalog number: 9115S01)
Microscopy slides #76 mm × 26 mm (ReliGlas, catalog number: 7101)
Gold-seal coverslips (22 mm2) (Electron Microscopy Sciences, catalog number: 63765-01)
Sucrose (SRL, catalog number: 84973)
Agar-agar (HiMedia, catalog number: GRM666)
Sugar-tolerant yeast (Angel, catalog number: 5331A201908)
Propionic acid (Merck, catalog number: 80060505001730)
WhatmanTM filter paper (GE Healthcare, catalog number: 1001917)
Paraformaldehyde (PFA) pH 7.4 (Sigma-Aldrich, catalog number: I58127)
Phosphate buffered saline (PBS) pH 7.4 (HiMedia, catalog number: ML023)
Triton X-100 (Sigma-Aldrich, catalog number: T8787)
Normal goat serum (NGS) (Vector Labs, catalog number: S1000)
Rabbit anti-tyrosine hydroxylase (anti-TH) polyclonal primary ab (Millipore, catalog number: Ab152)
Goat anti-rabbit IgG H&L (TRITC-labeled) polyclonal secondary ab (Abcam, catalog number: Ab6718)
VECTASHIELD® mounting medium (Vector Labs, catalog number: H1000)
Solutions
4% PFA solution (50 mL) (see Recipes)
0.1% PBST (phosphate buffered saline and Triton X-100) (50 mL) (see Recipes)
0.5% PBST (50 mL) (see Recipes)
5% NGS blocking buffer solution (1 mL) (see Recipes)
Anti-TH polyclonal primary ab solution (see Recipes)
TRITC-labeled polyclonal secondary ab solution (see Recipes)
Recipes
4% PFA solution (50 mL)
PFA 2 g
1× PBS 50 mL
Add PFA in 1× PBS in a conical flask, cover it with parafilm, and shake it thoroughly for 10 min.
Transfer the flask with a magnetic stirrer on the hotplate for heating/boiling with a temperature ranging from 80 °C to 110 °C with moderate stirring at 150 rpm.
Keep the flask on the hotplate until the cloudy solution becomes transparent.
After this, switch off the hotplate but keep the stirring for 15 min. Allow the solution to cool down, aliquot it in a 1.5 mL centrifuge tube, and store it at -80 °C.
Critical: Do not store the solution for more than a week.
Caution: PFA is a potential carcinogen; hence, the whole process should be done under a fume hood. Wear hand gloves and a lab coat during handling and preparation of PFA solution.
0.1% PBST (phosphate buffered saline and Triton X-100) (50 mL)
10× PBS 5 mL
Autoclaved enzyme-free water 45 mL
Triton X-100 50 μL
Add 5 mL of 10× PBS in 45 mL of autoclaved enzyme-free water.
Mix 50 μL of Triton X-100 and vortex it for 10 seconds. The solution can be stored at room temperature for one week.
0.5% PBST (50 mL)
10× PBS 5 mL
Autoclaved enzyme-free water 45 mL
Triton X-100 250 μL
Add 5 mL of 10× PBS in 45 mL of autoclaved enzyme-free water.
Mix 250 μL of Triton X-100 and vortex it for 10 s. The solution can be stored at room temperature for one week.
5% NGS blocking buffer solution (1 mL)
NGS 50 μL
0.5% PBST 950 μL
Add 50 μL of NGS in 950 μL of 0.5% PBST and mix it properly by vortexing for 10 s. The solution can be stored at room temperature for 1–2 h.
Anti-TH polyclonal primary ab solution
Anti-TH polyclonal primary ab 5 μL
5% NGS blocking buffer 1,245 μL
Take 1,245 μL of 5% NGS blocking buffer and add 5 μL of anti-TH polyclonal primary ab (1:250 dilution). Mix it gently by inverting the tube slowly and place it on the ice until used.
TRITC-labeled polyclonal secondary ab solution
TRITC-labeled polyclonal secondary ab 5 μL
5% NGS blocking buffer 1,245 μL
Take 1,245 μL of 5% NGS and add 5 μL of TRITC-labeled polyclonal secondary ab (1:250 dilution). Mix it gently by inverting the tube slowly and store it on ice until used.
Equipment
Fly head capsule handling items e.g., needles #31 G × 6 mm (Tentabe BD, catalog number: 324902)
Dissecting fine forceps (EMS, catalog number: 78620-4B)
Brush (TEYUP, model number: SR-1013)
Delicate task Kim wipers (KIMTECHTM, catalog number: 370080)
Micropipette i.e., 1,000 μL, 50 μL, 10 μL, 2 μL (Gilson, catalog number: 30040)
Frost-free refrigerator (Whirlpool, model number: FF26 4S)
pH/mV meter (Hanna Instruments, model: HI2211-02)
-20 °C ES Series refrigerator (Thermo Scientific, model: 50616100444443250)
-80 °C ultra-low temperature freezer (New Brunswick Innova, model: U101-86)
Stereo zoom microscope (Carl Zeiss, model: Stemi 305)
Stereo zoom microscope (Leica, model: E24)
Fume hood (BIOMATRIX, Telangana, India)
BOD incubator (Percival, model: DR-36VL)
Test tube rotator (Tarsons, Rotospin, catalog number: 3070) and disk for 24 × 1.5 mL tube (Tarsons, catalog number: 3071)
Axio Imager M2 fluorescence microscope fitted with 100 W Mercury lamp (Carl Zeiss, catalog number: 430004-9902-000)
AxioCam ICm1 monochromatic camera (Carl Zeiss, catalog number: 426553-9901-000)
Software and datasets
ZEN 2012 SP2 blue edition, version 2.0.14283.302 (Carl Zeiss, Jena, Germany)
Microsoft Office Excel Worksheet 2007 (Microsoft Inc., WA, USA)
GraphPad Prism, March 7, 2007, version 5.00 (GraphPad Inc., MA, USA)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Chaurasia, R., Ayajuddin, M., Ratnaparkhi, G. S., LS, S. and Yenisetti, S. C. (2024). A Simple Immunofluorescence Method to Characterize Neurodegeneration and Tyrosine Hydroxylase Reduction in Whole Brain of a Drosophila Model of Parkinson’s Disease. Bio-protocol 14(4): e4937. DOI: 10.21769/BioProtoc.4937.
分类
神经科学 > 神经系统疾病 > 帕金森氏症
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link