- EN - English
- CN - 中文
Use of the Fluorescent Dye Thioflavin T to Track Amyloid Structures in the Pathogenic Yeast Candida albicans
使用荧光染料硫磺素 T 追踪致病性酵母白色念珠菌中的淀粉样结构
(§ Technical contact: thierry.mourer@pasteur.fr) 发布: 2024年02月05日第14卷第3期 DOI: 10.21769/BioProtoc.4932 浏览次数: 2338

相关实验方案
在细菌中利用取代半胱氨酸可及性方法研究硫酯酰基中间体膜生化酶的拓扑结构和活性
Sébastien Gélis-Jeanvoine and Nienke Buddelmeijer
2015年11月05日 8782 阅读
利用合理化诱变和X射线晶体学分析在不同功能状态下的信号复合体DesK:DesR
Juan Andres Imelio [...] Alejandro Buschiazzo
2017年08月20日 8210 阅读
Abstract
The human pathogenic yeast Candida albicans can attach to epithelial cells or indwelling medical devices to form biofilms. These microbial communities are highly problematic in the clinic as they reduce both sensitivity to antifungal drugs and detection of fungi by the immune system. Amyloid structures are highly organized quaternary structures that play a critical role in biofilm establishment by allowing fungal cells to adhere to each other. Thus, fungal amyloids are exciting targets to develop new antifungal strategies. Thioflavin T is a specific fluorescent dye widely used to study amyloid properties of target proteins in vitro (spectrophotometry) and in vivo (epifluorescence/confocal microscopy). Notably, thioflavin T has been used to demonstrate the ability of Als5, a C. albicans adhesin, to form an amyloid fiber upon adhesion. We have developed a pipeline that allows us to study amyloid properties of target proteins using thioflavin T staining in vitro and in vivo, as well as in intact fungal biofilms. In brief, we used thioflavin T to sequentially stain (i) amyloid peptides, (ii) recombinant proteins, (iii) fungal cells treated or not with amyloid peptides, (iv) fungal amyloids enriched by cell fractionation, and (v) intact biofilms of C. albicans. Contrary to other methods, our pipeline gives a complete picture of the amyloid behavior of target proteins, from in vitro analysis to intact fungal biofilms. Using this pipeline will allow an assessment of the relevance of the in vitro results in cells and the impact of amyloids on the development and/or maintenance of fungal biofilm.
Key features
• Study of amyloid properties of fungal proteins.
• Visualization of the subcellular localization of fungal amyloid material using epifluorescence or confocal microscopy.
• Unraveling of the amyloid properties of target proteins and their physiological meaning for biofilm formation.
• Observation of the presence of amyloid structures with live-cell imaging on intact fungal biofilm using confocal microscopy.
Graphical overview
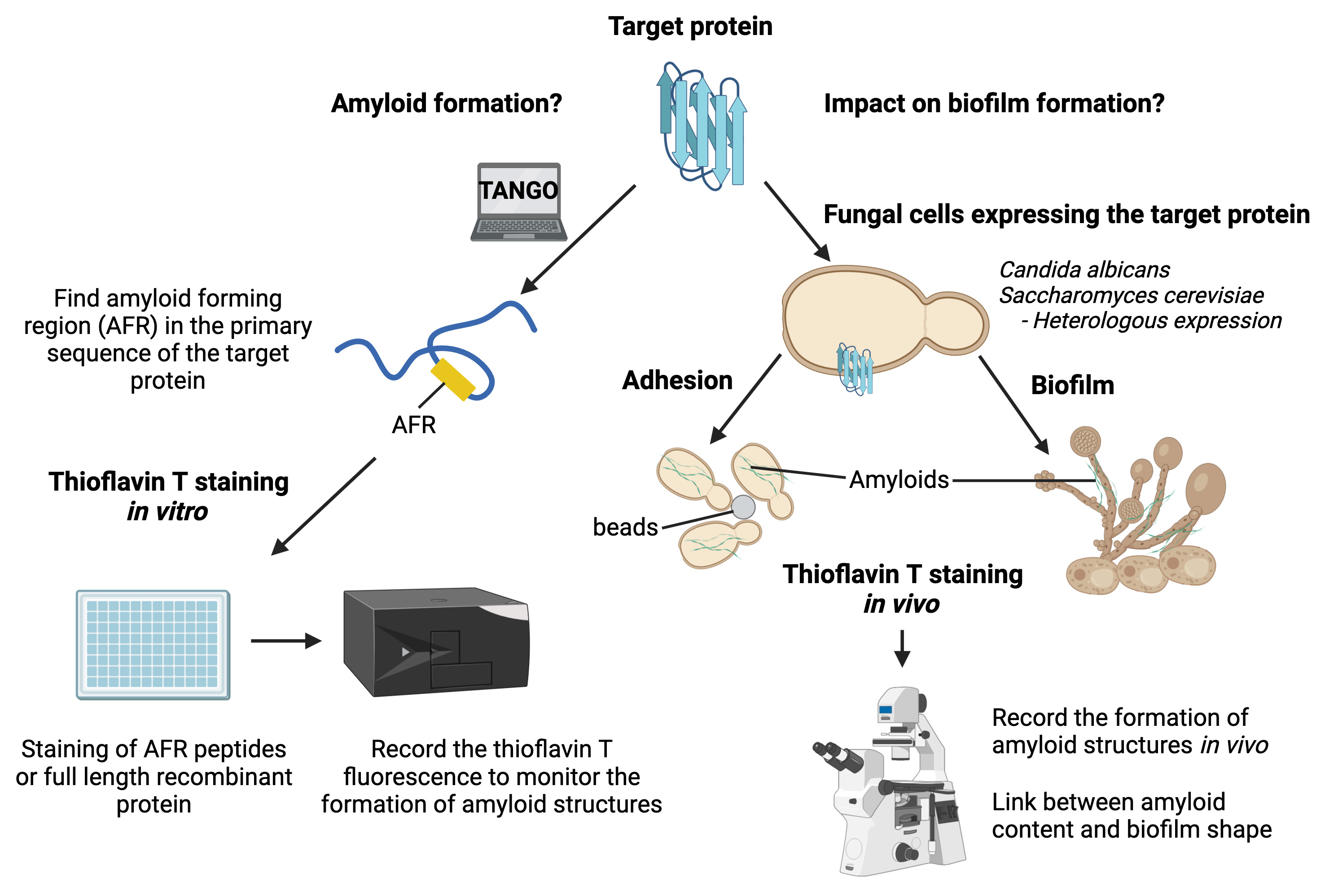
Background
Under certain circumstances (e.g., broad-spectrum antibiotics, neutropenia, abdominal surgery, or central venous catheter), the human fungal pathogen Candida albicans can be translocated through the gastrointestinal mucosa to reach the bloodstream and colonize organs such as kidneys or the brain (Pfaller and Diekema, 2007). Upon host colonization, C. albicans can form a highly structured microbial community named biofilm (Cabral et al., 2014). Biofilm formation starts with adhesion of cells on biotic or abiotic surfaces (Nobile and Johnson, 2015). Then, hyphae are extruded and elongated from the mother cells before the synthesis of the extracellular matrix. Once the matrix covers the fungal community, yeast cells are released from the biofilm, allowing new areas to be colonized. C. albicans biofilms are difficult to cure, as such structures reduce both sensitivity to antifungal agents and pathogen recognition by the host immune system (Brown et al., 2012). The cell wall plays a critical role in fungal biofilm establishment by allowing C. albicans cells to interact with their environment as well as maintaining the biofilm integrity. Over the past decade, multiple reports have shown that amyloid structures present in the microorganisms’ cell wall create adhesion forces between cells that promote biofilm formation (Ho et al., 2019). We have recently uncovered that Pga59, a cell wall protein, forms amyloid structures to promote cell–cell adhesion forces and hence contribute to biofilm formation in C. albicans (Mourer et al., 2023). To draw these conclusions, we took advantage of the amyloid-specific fluorescent dye thioflavin T (ThT). Specific binding of ThT to amyloid structures results in a shift of its emission wavelength and in an increase of its quantum yield. Hence, ThT is a powerful tool used to detect amyloid components in biological specimens. Regarding fungal biofilms, some cell wall proteins have been positively stained by ThT, thus demonstrating their ability to adopt the shape of amyloid structure (Mourer et al., 2023; Ramsook et al., 2010). However, the link between in vitro experiments and the fact that amyloid properties of proteins are important for biofilm formation or establishment is often missing. We developed a pipeline to explore the amyloid properties of target proteins as well as their relevance for fungal cell adhesion and biofilm formation. We used ThT to successively stain octapeptides, recombinant proteins, amyloid structures enriched from fungal cells, adherent cells treated or not with amyloid peptides, and finally intact biofilms. Using this pipeline can formally confirm or reject that 1) the target protein is able to form an amyloid structure and 2) this amyloid is physiologically relevant for biofilm formation. This protocol has the potential to be applied to other microorganisms that can form biofilms like the bacteria Pseudomonas aeruginosa or Staphylococcus epidermidis and hence, create new knowledge regarding the impact of amyloid proteins on biofilm formation.
Materials and reagents
Biological materials
Candida albicans BWP17 (Wilson et al., 1999)
Saccharomyces cerevisiae BY4742 (Winston et al., 1995)
Reagents
Thioflavin T (Sigma-Aldrich, catalog number: T3516-5g)
Dimethylsulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855-250 mL)
M-280 Dynabeads, tosyl-activated magnetic beads (Thermo Fisher Scientific, catalog number: 14204)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9418)
Concanavalin A, Alexa FluorTM 594 conjugate (Thermo Fisher Scientific, catalog number: C11253)
RNase A solution (Promega, catalog number: A7973)
TritonTM X-100 (Sigma-Aldrich, catalog number: X100-100ML)
Sucrose (Sigma-Aldrich, catalog number: S0389-500G)
10% CriterionTM XT Bis-Tris protein gel (Bio-Rad, catalog number: 3450112)
Bio-Rad protein assay dye reagent concentrate (Bradford) (Bio-Rad, catalog number: 5000006)
Poly-L-lysine solution (Sigma-Aldrich, catalog number: P8920)
Aclar® embedding film (TED PELLA, INC, catalog number: 10501-10)
GibcoTM RPMI 1640 medium (Thermo Fisher Scientific, catalog number: 10379144)
Dulbecco’s phosphate buffered saline (PBS) (Thermo Fisher Scientific, catalog number: 11590476)
Ethanol absolute (Merck, catalog number: 1009831011)
Magnesium chloride (Sigma-Aldrich, catalog number: 208337-100G)
Nonidet P-40 (Sigma-Aldrich, catalog number: I8896)
cOmplete, EDTA-free protease inhibitor tablets (Roche, catalog number: 11873580001)
Phenylmethylsulfonyl fluoride (PMSF) (Thermo Fisher Scientific, catalog number: 36978)
Glycerol (Sigma-Aldrich, catalog number: G5516-100ML)
Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: 62862)
Dithiothreitol (Sigma-Aldrich, catalog number: D0632-1G)
Tris hydrochloride (Thermo Fisher Scientific, catalog number: 15893661)
Sodium chloride (Sigma-Aldrich, catalog number: S9888-500G)
Bromophenol blue (Sigma-Aldrich, catalog number: B0126-25G)
Bradford reagent (Bio-Rad, catalog number: 500205)
BactoTM yeast extract (Thermo Fisher Scientific, catalog number: 212750)
BactoTM peptone (Thermo Fisher Scientific, catalog number: 211677)
Dextrose (Sigma-Aldrich, catalog number: D9434-1KG)
Yeast nitrogen base without amino acids (Sigma-Aldrich, catalog number: Y0626-1KG)
Glucose (Sigma-Aldrich, catalog number: G8270-1KG)
Arginine (Sigma-Aldrich, catalog number: A5006-100G)
Uridine (Sigma-Aldrich, catalog number: U3750-25G)
Histidine (Merck, catalog number: H3911)
Yeast synthetic drop-out medium supplements without uracil (Sigma-Aldrich, catalog number: Y1501-20G)
Solutions
Thioflavin T solution (ThT) (see Recipes)
Thioflavin T buffer (see Recipes)
Lysis buffer (see Recipes)
Amyloid-Prion resuspension buffer (see Recipes)
Amyloid-Prion buffer R (see Recipes)
YPD medium (see Recipes)
SD complete medium (see Recipes)
SC-Ura medium (see Recipes)
Recipes
Thioflavin T solution (3.3 mM)
Reagent Final concentration Quantity Thioflavin T 3.3 mM 10.53 mg Absolute ethanol n/a 10 mL Total n/a 10 mL Protect the thioflavin T solution from light with aluminum foil and store the solution at -20 °C up to six months.
Thioflavin T buffer
Reagent Final concentration Quantity Tris hydrochloride (1 M, pH 8.0) 20 mM 200 μL Sodium chloride (5 M) 150 mM 300 μL Thioflavin T (3.3 mM) 40 μM 121 μL Distilled H2O n/a 9.4 mL Total n/a 10 mL Prepare the thioflavin T buffer freshly before each experiment.
Lysis buffer
Reagent Final concentration Quantity Tris hydrochloride (1 M, pH 7.5) 50 mM 750 μL Sodium chloride (5 M) 150 mM 450 μL Magnesium chloride (1 M) 5 mM 75 μL Nonidet P-40 0.1% 15 μL PMSF (200 mM) 1 mM 75 μL cOmplete protease inhibitor n/a 2 tablets Distilled H2O n/a 13.6 mL Total n/a 15 mL Store the lysis buffer without protease inhibitors (PMSF and cOmplete protease inhibitor tablets) at 4 °C for two months maximum. Add protease inhibitors freshly before each experiment.
Amyloid-Prion resuspension buffer
Reagent Final concentration Quantity Tris hydrochloride (1 M, pH 7.5) 50 mM 350 μL Sodium chloride (5 M) 100 mM 140 μL SDS (20%) 2% 700 μL Dithiothreitol (1 M) 5 mM 35 μL Glycerol 5% 350 μL cOmplete protease inhibitor n/a 1 tablet Distilled H2O n/a 5.4 mL Total n/a 7 mL Store the Amyloid-Prion resuspension buffer for eight weeks at 4 °C without dithiothreitol and protease inhibitor. Both the dithiothreitol and the protease inhibitor tablet should be added just before the start of the experiment.
Amyloid-Prion buffer R
Reagent Final concentration Quantity Tris hydrochloride (1 M, pH 7.5) 10 mM 100 μL SDS (20%) 0.4% 200 μL Dithiothreitol (DTT, 1 M) 5 mM 50 μL Distilled H2O n/a 9.7 mL Total n/a 10 mL The amyloid-prion buffer R can be kept at room temperature for three months. Add the dithiothreitol just before starting the experiment.
YPD medium
Reagent Final concentration Quantity BactoTM yeast extract 1% 10 g BactoTM peptone
Dextrose
Distilled H2O
2%
2%
n/a
20 g
20 g
q.s. to 1 L
Total n/a 1 L SD complete medium
Reagent Final concentration Quantity Yeast nitrogen base without amino acids 0.67% 6.7 g Glucose
Arginine (2 mg/mL)
Uridine (4 mg/mL)
2%
20 mg/mL
40 mg/mL
20 g
10 mL
10 mL
Histidine (2 mg/mL) 20 mg/mL 10 mL Distilled H2O n/a q.s. to 1 L Total n/a 1 L SC-Ura medium
Reagent Final concentration Quantity Yeast nitrogen base without amino acids 0.67% 6.7 g Glucose 2% 20 g Yeast synthetic drop-out medium supplements without uracil 0.1% 1 g Distilled H2O n/a q.s. to 1 L Total n/a 1 L
Laboratory supplies
1.5 mL Eppendorf tubes (Eppendorf, catalog number: 0030125207)
50 mL FalconTM tubes (Fisher Scientific, catalog number: 10203001)
TPP® 96-well plate (Merck, catalog number: Z707902-108EA)
Culture plate 12 wells TPP (Dutscher, catalog number: 109212)
1.5 mL screw-cap micro tubes (Fisher Scientific, catalog number: 11549924)
Glass beads 0.5 mm, acidic wash (Sigma-Aldrich, catalog number: G8772-100 G)
Petri dishes 35 mm (Greiner, catalog number: P5112)
Ultracentrifuge polypropylene tubes (Beckman-Coulter, catalog number: 331372)
Glass slide (Merck, catalog number: S9027-1CS)
Coverslip (VWR, catalog number: 43210.KG)
Centrifuge bottles (Beckman-Coulter, catalog number: C31600)
Plastic cuvettes BRANDTM (Fisher Scientific, catalog number: 10566581)
Equipment
Microplate reader (TECAN, model: Infinite® 200 PRO)
Microcentrifuge (Eppendorf, catalog number: Z606235)
Ultracentrifuge OPTIMA XPN (Beckman-Coulter, catalog number: A94468)
Upright confocal microscope (Zeiss, model: LSM700)
ThermoMixer® C (Eppendorf, catalog number: 5382000015)
Sorvall RC-5B centrifuge (Thermo Fisher Scientific, catalog number: sorvall-RC-5B)
Vortex mixer (VWR, catalog number: 444-1372)
Centrifuge 5810 (Eppendorf, catalog number: 5810000010)
Microscope (Zeiss, model: Epifluorescence ApoTome)
Spectrophotometer (GE UltrospecTM 2100 pro, catalog number: GE80211221)
Bullet Blender Storm Pro (Next Advance, catalog number: 152081)
2 L Erlenmeyer flask (VWR, catalog number: 10545-844)
Electrophoresis power supply (Bio-Rad, model: 200/2.0)
Mini trans-blot electrophoretic transfer cell (Bio-Rad, catalog number: 1703930)
Water bath (Cole-Parmer, catalog number: WB-300-15)
Benchtop tube rotator RotoFlex (Cole-Parmer, catalog number: 120VAC)
Multitron shaker (Infors HT)
Software and datasets
Fiji ImageJ software (https://imagej.net/software/fiji/downloads)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Mourer, T., d’Enfert, C. and Bachellier-Bassi, S. (2024). Use of the Fluorescent Dye Thioflavin T to Track Amyloid Structures in the Pathogenic Yeast Candida albicans. Bio-protocol 14(3): e4932. DOI: 10.21769/BioProtoc.4932.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 结构
生物化学 > 蛋白质 > 荧光
微生物学 > 微生物生物膜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










