- EN - English
- CN - 中文
Engineering a CRISPRoff Platform to Modulate Expression of Myeloid Cell Leukemia (MCL-1) in Committed Oligodendrocyte Neural Precursor Cells
设计 CRISPRoff 平台以调节骨髓细胞白血病(MCL-1)在定向少突胶质细胞神经前体细胞中的表达
(§ Technical contact) 发布: 2024年01月05日第14卷第1期 DOI: 10.21769/BioProtoc.4913 浏览次数: 2655
评审: Salma MerchantVishal Nehru
Abstract
In vitro differentiation of human pluripotent stem cell (hPSC) model systems has furthered our understanding of human development. Techniques used to elucidate gene function during early development have encountered technical challenges, especially when targeting embryonic lethal genes. The introduction of CRISPRoff by Nuñez and collaborators provides an opportunity to heritably silence genes during long-term differentiation. We modified CRISPRoff and sgRNA Sleeping Beauty transposon vectors that depend on tetracycline-controlled transcriptional activation to silence the expression of embryonic lethal genes at different stages of differentiation in a stable manner. We provide instructions on how to generate sgRNA transposon vectors that can be used in combination with our CRISPRoff transposon vector and a stable hPSC line. We validate the use of this tool by silencing MCL-1, an anti-apoptotic protein, which results in pre-implantation embryonic lethality in mice; this protein is necessary for oligodendrocyte and hematopoietic stem cell development and is required for the in vitro survival of hPSCs. In this protocol, we use an adapted version of the differentiation protocol published by Douvaras and Fossati (2015) to generate oligodendrocyte lineage cells from human embryonic stem cells (hESCs). After introduction of the CRISPRoff and sgRNAs transposon vectors in hESCs, we silence MCL-1 in committed oligodendrocyte neural precursor cells and describe methods to measure its expression. With the methods described here, users can design sgRNA transposon vectors targeting MCL-1 or other essential genes of interest to study human oligodendrocyte development or other differentiation protocols that use hPSC model systems.
Key features
• Generation of an inducible CRISPRoff Sleeping Beauty transposon system.
• Experiments performed in vitro for generation of inducible CRISPRoff pluripotent stem cell line amenable to oligodendrocyte differentiation.
• Strategy to downregulate an essential gene at different stages of oligodendrocyte development.
Graphical overview
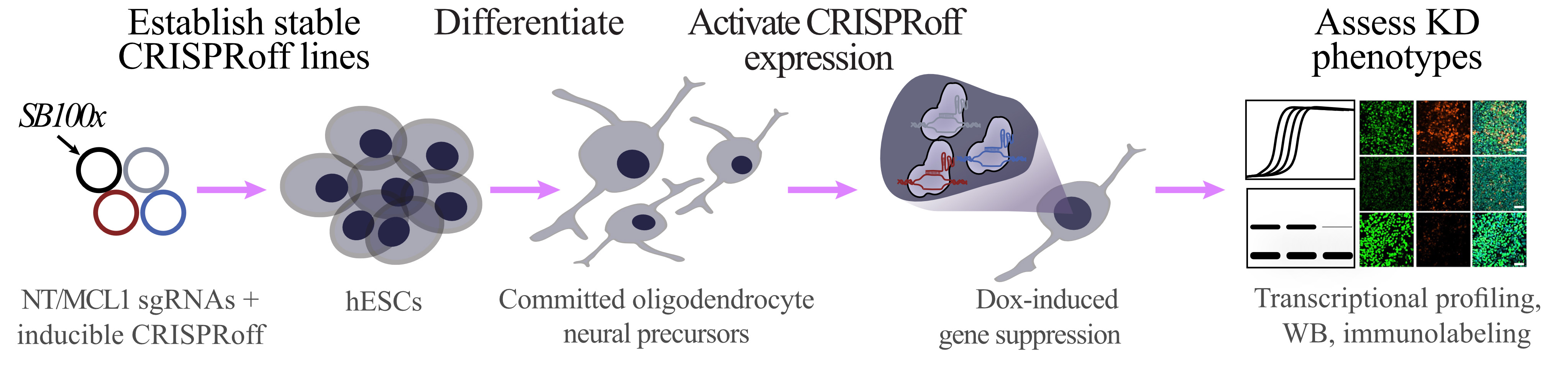
Workflow for generating inducible CRISPRoff stem cell line and assessing knockdown phenotype in stem cell–derived committed oligodendrocyte neural precursor cells
Background
In vitro systems of differentiation have become useful tools to dissect the molecular mechanisms involved in cell fate transitions. The use of human pluripotent stem cells (PSCs) has revolutionized our understanding of early stages of human development that were previously inaccessible. Different gene-targeting techniques, such as RNAi, TALEN, Cas9 nuclease, and CRISPRi/a, are available to investigate the function of specific genes during development [1–3]. Partial gene knockdowns in PSCs are commonly used to study the function of genes that would be lethal if completely knocked out. However, most of these tools have transient genetic modifications and their effectiveness is diminished during in vitro differentiation of PSCs. Nuñez et al. pioneered CRISPRoff, a programmable epigenetic memory writer protein that can heritably silence genes [4]. This tool has greatly benefited the field, allowing developmental researchers to further understand the function of genes during long-term in vitro differentiations, as epigenetic memory is stable. We built on the CRISPRoff system by adding a Sleeping Beauty transposon system containing a reverse tetracycline-controlled transactivator (Figure 1A). Additionally, we designed a single-guide RNA (sgRNA) transposon plasmid that allows for efficient genomic integration (Figure 1B). With the CRISPRoff and sgRNA transposon vectors described here, we were able to develop a novel experimental approach to inducibly silence expression of MCL-1, an anti-apoptotic protein, in oligodendrocyte lineage cells.
Deletion of Mcl-1 results in pre-implantation embryonic lethality [5]. Consequently, investigating the function of MCL-1 during development has barriers as mouse embryos are not able to implant and PSCs undergo apoptosis once MCL-1 is knocked down or inhibited via small molecules [6, 7]. This technical barrier has halted further research on the function of MCL-1 during early human neurodevelopment. With CRISPRoff, we silenced expression of MCL-1 at different stages of neurodevelopment. In this protocol, we outline how this set of tools can be used to investigate the potential function of MCL-1 during development of oligodendrocyte lineage cells. We adapted this technology and linked it to an established oligodendrocyte generation protocol [8] to validate the use of these novel tools during differentiation of human PSCs. Moreover, we detail how to generate sgRNA transposon vectors for other genes of interest that can be adapted into alternative differentiation systems.
There is a lack of literature that combines culturing of human oligodendrocyte lineage cells with epigenetic editing strategies. This protocol aims to bridge that gap by using a proof-of-principle experiment where MCL-1 is silenced at an early stage of differentiation. This protocol can be used to investigate an array of genes that cause lethality in PSCs or for studying gene function at different stages of oligodendrocyte development.
Materials and reagents
Biological materials
H9 human embryonic stem cells (WiCell Research Institute, WA09, NIH Registration Number: 0062)
Competent E. coli cells (New England Biolabs, catalog number: C2987H/C2987I)
pSB-TRE-CRISPRoff-EF1A-TetOn (Addgene, catalog number: 203355)
pSB-BbsI-sgRNA (Addgene, catalog number: 203359)
pCMV(CAT)T7-SB100 (Addgene, catalog number: 34879)
Reagents
BbsI-HF endonuclease (New England Biolabs, catalog number: R0539/R0539L)
rCutSmart buffer (New England Biolabs, catalog number: B6004S)
Quick calf intestinal alkaline phosphatase (CIP) (New England Biolabs, catalog number: M0525S/M0525L)
PCR and/or gel extraction purification kit (Invitrogen, catalog number: K220001)
T4 DNA ligase reaction buffer (New England Biolabs, catalog number: B0202S)
T4 DNA ligase (New England Biolabs, catalog number: M0202S/M0202L)
Polynucleotide kinase (PNK) (New England Biolabs, catalog number: M0201S/M0201L)
Recommended colony PCR reagents include Taq DNA Polymerase with ThermoPol® buffer (New England Biolabs, catalog number: M0267S/M0267L) and deoxynucleotide (dNTP) solution mix (New England Biolabs, catalog number: N0447S/N0447L)
DifcoTM LB broth, Miller (Luria-Bertani) (Becton Dickinson, catalog number: 244610)
LB agar, Miller (Fisher Scientific, catalog number: BP1425-500/BP1425-2)
Plasmid DNA Miniprep kit (Qiagen, catalog number: 27104)
Ampicillin sodium salt (Thermo Fisher Scientific, catalog number: 611770250/611770050)
TRIzolTM reagent (Thermo Fisher Scientific, catalog number: 15596026)
RNaseZAP (Thermo Fisher Scientific, catalog number: AM9780)
Chloroform (Millipore Sigma, catalog number: C2432)
2-Propanol (Millipore Sigma, catalog number: I9516)
Ethyl alcohol (Millipore Sigma, catalog number: 459836)
Diethyl pyrocarbonate (DEPC)-treated water (KD Medical, catalog number: RGE3050)
Ethylenediaminetetraacetic acid (EDTA), 0.5 M, pH 8.0, molecular biology grade, DEPC-treated (Millipore Sigma, catalog number: 324506)
DNase I (New England Biolabs, catalog number: M0303)
Thermo Fisher High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, catalog number: 4368814)
SYBR Green PCR Master Mix (Thermo Fisher Scientific, catalog number: 4309155)
Nuclease-free water (Thermo Fisher Scientific, catalog number: AM9906)
Phosphate-buffered saline (PBS) tablets (Fisher BioReagents, catalog number: BP2944100)
Paraformaldehyde (PFA) 16% aqueous solution (Electron Microscope Sciences, catalog number: 15710-S)
Triton X-100 (Millipore Sigma, catalog number: T87876)
Bovine serum albumin (BSA) (Millipore Sigma, catalog number: A4503-100G)
Fluoromount-G (Thermo Fisher Scientific, catalog number: 00-4958-02)
Sodium dodecyl sulfate (SDS) (Millipore Sigma, catalog number: L3771)
Trizma® base (Tris Base) (Millipore Sigma, catalog number: T6066)
Glycine (Millipore Sigma, catalog number: G7126)
Sodium chloride (NaCl) (Millipore Sigma, catalog number: S7653)
Hydrochloric acid (HCl) (Millipore Sigma, catalog number: 258148)
Dry powder milk (RPI, catalog number: M17200)
TWEEN® 20 (Millipore Sigma, catalog number: P7949)
Phosphatase inhibitor PhosSTOP (Roche, Thermo Fisher Scientific, catalog number: 4906845001)
Protease inhibitor phenylmethylsulfonyl fluoride (PMSF) (Roche, Thermo Fisher Scientific, catalog number: 10837091001)
cOmpleteTM, EDTA-free protease inhibitor cocktail (PIC) (Roche, Thermo Fisher Scientific, catalog number: 4693132001)
Pierce bicinchoninic acid (BCA) Protein Assay kit (Thermo Fisher Scientific, catalog number: 23227)
4× BlotTM lithium dodecyl sulfate (LDS) sample buffer (Thermo Fisher Scientific, catalog number: B0007)
2-Mercaptoethanol (Bio-Rad, catalog number: 1610710)
Hoechst 33342 solution (Thermo Fisher Scientific, catalog number: 62249)
4%–20% Mini-PROTEAN® TGXTM Precast protein gels (Bio-Rad catalog number: 4561091)
mTeSR1 (STEMCELL Technologies, catalog number: 85850)
Matrigel Matrix hESC-qualified mouse (Corning, catalog number: 354277)
DMEM/F-12 (N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid) HEPES (Thermo Fisher Scientific, catalog number: 11330032)
Gentle cell dissociation reagent (STEMCELL Technologies, catalog number: 100-0485)
ROCK1 and ROCK2 inhibitor Y-27632 (STEMCELL Technologies, catalog number: 72307)
Opti-MEM I reduced serum medium (Thermo Fisher Scientific, catalog number: 31985070)
TransIT-LT1 transfection reagent (Mirus, catalog number: 2304)
Accutase (Innovative Cell Technologies, catalog number: AT104)
Puromycin dihydrochloride from Streptomyces alboniger (Millipore Sigma, catalog number: P8833-10MG)
Hygromycin B (Thermo Fisher Scientific, catalog number: 10687010)
Caspase inhibitor Quinoline-Val-Asp-Difluorophenoxymethylketone (Q-VD-Oph) (SM Biochemicals LLC, catalog number: SMP001-5MG)
Doxycycline hyclate (Tocris Bioscience, catalog number: 4090)
Insulin solution human (Thermo Fisher Scientific, catalog number: I9278)
DMEM/F-12 (Thermo Fisher Scientific, catalog number: 11320033)
Penicillin-Streptomycin (Thermo Fisher Scientific, catalog number: 15140122)
MEM non-essential amino acids solution 100× (Thermo Fisher Scientific, catalog number: 11140050)
GlutaMAX supplement (Thermo Fisher Scientific, catalog number: 35050061)
Retinoic acid (Millipore Sigma, catalog number: R2625)
TGF-beta/Smad inhibitor LDN-193189 (Reprocell, catalog number: 04-0074)
TGF-beta/Smad inhibitor Stemolecule SB431542 (Reprocell, catalog number: 04-0010-10)
N-2 supplement 100× (Thermo Fisher Scientific, catalog number: 17502048)
Smoothened agonist (SAG) (STEMCELL Technologies, catalog number: 73412)
Ultrapure DNase/RNase-free distilled water (Thermo Fisher Scientific, catalog number: 10977015)
Solutions
DNase I reaction (see Recipes)
Reverse transcription master mix (see Recipes)
1% Triton (see Recipes)
4% PFA (see Recipes)
10% BSA (see Recipes)
Lysis buffer (see Recipes)
10× Tris-Gly-SDS buffer (see Recipes)
20× TBS (see Recipes)
TBST (see Recipes)
5% Milk in TBST (see Recipes)
Basal medium (see Recipes)
Neural induction medium (NIM) (see Recipes)
N2 medium (see Recipes)
Recipes
DNase I reaction
Note: Keep on ice and use immediately after preparation.
Reagent Final concentration Quantity or Volume DNase I reaction buffer (10×) 1× 1 µL DNase I (RNase-free) n/a 0.2 µL RNA sample n/a 2 µg Nuclease-free H2O n/a Up to 10 µL Total n/a 10 µL Reverse transcription master mix
Note: Keep on ice and use immediately after preparation.
Reagent Final concentration Quantity or Volume 10× RT buffer 1× 2 µL 25× dNTP mix 1× 0.8 µL 10× RT random primers 1× 2 µL MultiScribeTM reverse transcriptase n/a Up to 10 µL Nuclease-free H2O n/a 4.2 µL Total n/a 10 µL 1% Triton
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume Triton X-100 1% 50 µL 1× PBS n/a 49.5 mL 4% PFA
Reagent Final concentration Quantity or Volume 16% PFA 4% 12.5 mL 1× PBS n/a 37.5 mL 10% BSA
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume BSA 10% 5 g 1× PBS n/a 50 mL Lysis buffer
Note: Keep on ice and use immediately after preparation.
Reagent Final concentration Quantity or Volume 1% Triton (Recipe 3) 0.79% 79 µL 10× PhosSTOP 1× 10 µL 10× PIC 1× 10 µL 100 mM PMSF 1 mM 1 µL Total n/a 100 µL 10× Tris-Gly-SDS buffer
Reagent Final concentration Quantity or Volume Tris Base n/a 121.1 g Glycine n/a 576 g SDS 1% 200 mL ddH2O n/a Up to 4 L Total n/a 4 L 20× TBS
Note: Adjust pH to 7.6 with HCl.
Reagent Final concentration Quantity or Volume Tris Base n/a 193.6 g NaCl n/a 640 g ddH2O n/a Up to 4 L Total n/a 4 L TBST
Reagent Final concentration Quantity or Volume 20× TBS 1× 50 mL ddH2O n/a 949 mL TWEEN® 20 0.1% 1 mL Total n/a 1 L 5% Milk in TBST
Reagent Final concentration Quantity or Volume Dry powder milk 5% 5 g TBST n/a 100 mL Total n/a 100 mL Basal medium
Note: Filter sterilize and store at 4 °C.
Reagent Final concentration Quantity or Volume DMEM/F12 n/a 485 mL MEM non-essential amino acids solution 1× 5 mL GlutaMAX supplement 1× 5 mL Penicillin-Streptomycin 1× 5 mL 2-Mercaptoethanol 55 µM 1.93 µL Total n/a 500 mL Neural induction medium (NIM)
Note: Store at 4 °C. *Add small molecules fresh daily.
Reagent Final concentration Quantity or Volume Basal medium (Recipe 7) n/a 50 mL Insulin 25 µg/mL 108 µL SB431542* 10 µM 25 µL LDN193189* 250 nM 6.25 µL Retinoic acid* 100 nM 50 µL Total n/a 50 mL N2 medium
Note: Store at 4 °C. *Add small molecules fresh daily.
Reagent Final concentration Quantity or Volume Basal medium (Recipe 7) n/a 49.5 mL N-2 supplement 100× 1× 500 µL SAG* 250 nM 5 µL RA* 100 nM 50 µL Total n/a 50 mL
Laboratory supplies
1.5 mL microcentrifuge tubes (Fisherbrand, catalog number: 05-408-1317)
PCR tubes (Thermo Fisher Scientific, catalog number: AB2000)
Costar 6-well clear TC-treated well plates (Corning, catalog number: 3516)
Cell lifter (Corning, catalog number: 3008)
Surface-treated sterile tissue culture plates (Fisherbrand, catalog number: FB012928)
Stericup-GP 500 mL Express Plus (Millipore Sigma, catalog number: S2GPU05RE)
35 mm glass-bottom dish with 14 mm micro-well #1.5 cover glass (Cellvis, catalog number: D35-14-1.5-N)
Equipment
Applied BiosystemsTM QuantStudioTM 3 Real-Time PCR System, 96-well, 0.2 mL, laptop (Thermo Fisher Scientific, catalog number: A28567)
Thermocycler (Thermo Fisher Scientific, catalog number: 4484073)
Incubated shaker (Thermo Fisher Scientific, catalog number: SHKE6000)
Synergy HT Microtiter Plate reader (BioTek, catalog number: 7091000)
Amersham Imager 600 (General Electric, catalog number: AI600)
Stirring Hotplates (Thermo Fisher Scientific, catalog number: SP88854100)
Mini-PROTEAN Tetra Vertical Electrophoresis Cell (Bio-Rad, catalog number: 1658004
PowerPacTM HC power supply (Bio-Rad, catalog number: 1645052)
Spinning disk confocal microscope (Nikon Eclipse Ti-E equipped with a Plan Apo Lambda 20× 0.75 NA WD 1.00 mm objective and an Andor DU-897 EMCCD camera)
Software and datasets
Prism v9 (GraphPad, 10/24/2020)
Image Studio Lite
SnapGene v6.2.1
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Gil, M., Hamann, C. A., Brunger, J. M. and Gama, V. (2024). Engineering a CRISPRoff Platform to Modulate Expression of Myeloid Cell Leukemia (MCL-1) in Committed Oligodendrocyte Neural Precursor Cells. Bio-protocol 14(1): e4913. DOI: 10.21769/BioProtoc.4913.
分类
神经科学 > 细胞机理
生物工程 > 生物医学工程
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link