- EN - English
- CN - 中文
Quantifying Cell Proliferation Through Immunofluorescence on Whole-Mount and Cryosectioned Regenerating Caudal Fins in African Killifish
通过免疫荧光定量非洲鳉鱼整体和再生尾鳍冷冻切片的细胞增殖
发布: 2023年12月20日第13卷第24期 DOI: 10.21769/BioProtoc.4908 浏览次数: 2638
评审: Anna Sloutskin Ivonne SehringJohn W Peterson
Abstract
The African killifish Nothobranchius furzeri is an attractive research organism for regeneration- and aging-related studies due to its remarkably short generation time and rapid aging. Dynamic changes in cell proliferation are an essential biological process involved in development, regeneration, and aging. Quantifying the dynamics of cell proliferation in these contexts facilitates the elucidation of the attendant underlying mechanisms. Whole-mount and cryosectioning sample preparation are the preferred approaches to investigate the distribution of cellular structures, cell–cell communication, and spatial gene expression within tissues. Using African killifish caudal fin regeneration as an example, we describe an efficient and detailed protocol to investigate cell proliferation dynamics in both space and time during caudal fin regeneration. The quantification of cell proliferation was achieved through high-resolution immunofluorescence of the proliferation marker Phospho-Histone H3 (H3P). We focused on the characterization of epithelial and mesenchymal proliferation in three-dimensional space at two regeneration time points. Our protocol provides a reliable tool for comparing cell proliferation under different biological contexts.
Key features
• Elaborates in detail the method used by Wang et al. (2020) to quantify whole-organ mitotic events during tail fin regeneration in vertebrates.
• Enables proliferation analysis of millimeter-sized homeostatic and regenerating tissues.
• Three-day alternative method to whole mount using cryosections.
• Allows automatic quantification using ImageJ macros and R scripts.
Graphical overview
Background
Understanding the genetic and molecular basis of post-embryonic development in vertebrates, such as regeneration and aging, are long-standing quests in biology. One of the limiting steps in addressing such questions is the relative long generation time of most existing research organisms. As a result, identifying and characterizing phenotypes associated with adult stages is much more time-consuming when compared to embryonic stages. The emerging research organism African killifish, Nothobranchius furzeri, has received increasing attention in the fields of regeneration and aging due to its remarkable biology, including fast sexual maturation time and rapid aging (Genade et al., 2005; Harel et al., 2015; Hu et al., 2020; Wang et al., 2020). Quantifying the dynamic changes of cell proliferation is a critical procedure that has been frequently conducted in many different animal models (Kang and Sánchez Alvarado, 2009; Poleo et al., 2001; Wang et al., 2011).
Currently, cryosection and whole-mount sample preparations are efficient and generally accepted approaches to examine the dynamics of cell proliferation associated with a biological process of interest. The former allows for a quick analysis of a single plane in Z along the tissues, and high-resolution imaging of deep tissue structures is possible using this technique. In contrast, the whole-mount approach provides spatial information in 3D that is missing in cryosections at the expense of reduced ability for high-resolution imaging (usually requires short working distance lenses) due to the thickness of tested samples. Further, whole-mount samples retain all information on cell–cell interactions that are only apparent when looking at the whole structure. Integration of the two approaches makes it possible to characterize local features at high-resolution and perform three-dimensional analysis of biological markers of interest.
Phospho-histone 3 (H3P) has been widely used as a proliferation marker because this post-translational modification is deeply conserved across many species with a large phylogenetic distance (Newmark and Sánchez Alvarado, 2000; Nielsen et al., 2013; Wang et al., 2011). The epitope recognized by commercially available antibodies tends to be cross-reactive among wide ranges of species, making H3P the go-to mitosis marker in many research laboratories. In contrast to other proliferation markers such as Ki67 or EdU, H3P displays higher signal to noise ratios and its sparsity within the tissue allows for robust nuclei segmentation. In this protocol, we use the quantification of cell proliferation in killifish fin regeneration as an example to illustrate the challenges and discuss tips for successful H3P immunofluorescence on both cryosections and whole mounts in terms of tissue penetration, pigment bleaching, clearing, imaging, and data processing.
Materials and reagents
Common between whole mount and cryosections
16% Paraformaldehyde solution (Electron Microscopy Sciences, catalog number: 15710)
Methanol (MetOH) (Sigma-Aldrich, catalog number: 34860)
Monoclonal mouse antibody anti-E-Cadherin (Monoclonal Ecad Ab) (BD Biosciences, catalog number: 610182)
Monoclonal rabbit antibody anti-Phospho-Histone H3 (Ser10) (D2C8) (Monoclonal H3P Ab) (Cell Signaling Technology, catalog number: 3377S)
Polyclonal goat antibody anti-Rabbit IgG H&L Alexa Fluor 647 preadsorbed (Abcam, catalog number: ab150083) or F(ab')2-Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 647 (Thermo Fisher, catalog number: A48285)
Polyclonal goat antibody anti-Mouse IgG H&L Alexa Fluor 555 preadsorbed (Abcam, catalog number: ab150118) or F(ab')2-Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa FluorTM Plus 555 (Thermo Fisher, catalog number: A48287)
TWEEN® 20 (Sigma-Aldrich, catalog number: P1379)
100 mm Petri dish (Fisher Scientific, catalog number: FB0875712)
Horse serum, heat inactivated (Thermo Fisher, catalog number: 26050070)
Goat serum (Thermo Fisher, catalog number: 16210072)
Fetal bovine serum (FBS) (Thermo Fisher, catalog number: A5256801)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 472301)
Sodium azide (NaN3) (Sigma-Aldrich, catalog number: S2002)
Roche western blocking reagent solution (Sigma-Aldrich, catalog number: 11921673001)
Glycerol (Sigma-Aldrich, catalog number: G7893)
YOYO-1 iodide (491/509), 1 mM solution in DMSO (Thermo Fisher, catalog number: Y3601)
30 mL polypropylene jar (Fisher Scientific, catalog number: 02-891A)
MS-222 (Sigma-Aldrich, catalog number: E10521)
Formic acid (Sigma-Aldrich, catalog number: 695076)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S5886)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P3911)
Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S9763)
Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
500 mL bottle top vacuum filter, 0.22 µm pore (Corning, catalog number: 431118)
Whole mount
60 mm Petri dish (Fisher Scientific, catalog number: 08-757-100B)
Dumont forceps (Fisher Scientific, catalog number: NC9518792)
Sterile 50 mL conical tubes (Fisher Scientific, catalog number: 14-432-22)
10 mL pipette (Fisher Scientific, catalog number: 13-678-12E)
Razor blade (Fisher Scientific, catalog number: 12-640)
Transfer pipette (Fisher Scientific, catalog number: 13-711-5B)
Ibidi USA µ-dish coverslip bottom 35 mm high Petri dishes (Fisher Scientific, catalog number: 50-305-805)
White lamp with goose neck (Ikea JANSJÖ 3W lamp)
DER 332 (Sigma-Aldrich, catalog number: 31185)
DER 736 (Sigma-Aldrich, catalog number: 31191)
Isophorone diamine (IPDA) (Sigma-Aldrich, catalog number: 118184)
Hydrogen peroxide (H2O2) solution 30% (w/w) in H2O (Sigma-Aldrich, catalog number: H1009)
Dichloromethane (DCM) (Sigma-Aldrich, catalog number: 270997)
Dibenzyl ether (DBE) (Sigma-Aldrich, catalog number: 33630)
Corning 10 mL polystyrene serological pipette (Fisher Scientific, catalog number: 07-200-12)
Cryosections
30% Sucrose solution (Sigma-Aldrich, catalog number: S0389). Store the solution at 4 °C
FalconTM 15 mL conical centrifuge tubes (Fisher Scientific, catalog number: 14-959-49B)
FisherbrandTM disposable base molds (Thermo Fisher, catalog number: 22-363-552)
SuperFrost Plus microscope slides (Avantor, VWRI631-0108)
Super PAP pen (Thermo Fisher, catalog number: 008899)
TritonTM X-100 solution (Sigma-Aldrich, catalog number: 93443)
Reagents for bleaching:
H2O2 (Sigma-Aldrich, catalog number: 88597)
Formamide (Sigma-Aldrich, catalog number: 11814320001)
Optimal cutting temperature (OCT) embedding compound (SAKURA, catalog number: 4583)
TrueBlack lipofuscin (Biotium, catalog number: 23007)
ProLong Gold antifade mountant (Thermo Fisher Scientific, catalog number: P36930)
Tsugar memory miniature paint brushes (Amazon, catalog number: ASIN B0BJQ2CC8D)
DAPI solution 1 mg/mL (Thermo Fisher Scientific, catalog number: 62248)
200 proof ethanol (Sigma-Aldrich, catalog number: E7023)
Solutions
PBS-Tween (see Recipes)
Fixative solution (see Recipes)
10% Tween 20 (see Recipes)
PBS-Tween-NaN3 (see Recipes)
123 mM NaN3 (see Recipes)
Bleaching solution (see Recipes)
25% MetOH (see Recipes)
50% MetOH (see Recipes)
75% MetOH (see Recipes)
Blocking solution (see Recipes)
Primary antibody solution (see Recipes)
Secondary antibody solution (see Recipes)
Secondary antibody stock solution (see Recipes)
Nuclear dye solution (see Recipes)
DCM-MetOH (see Recipes)
DAPI solution (see Recipes)
Phosphate-buffered saline (PBS 1× pH 7.4) (see Recipes)
TrueBlack solution (see Recipes)
Recipes
PBS-Tween
Reagents Final concentration Quantity Unit 10% Tween 20 (Recipe 3) 0.1% (0.89 mM) 10 mL PBS 1× pH 7.4 1× 990 mL Final volume 1,000 mL Note: No need to adjust pH.
Fixative solution
Reagents Final concentration Quantity Unit 16 % paraformaldehyde 4% (1.17 M) 10 mL Formic acid 0.5% (132 mM) 200 μL PBS-Tween (1×) (Recipe 1) 1× 29.8 mL Final volume 40 mL Note: No need to adjust pH.
10% Tween 20
Reagents Final concentration Quantity Unit Tween 20 10% (89 mM) 10 mL dH2O 90 mL Final volume 100 mL Note: Filter solution (0.22 μm pore filter). Store at 4 °C. No need to adjust pH.
PBS-Tween-NaN3
Reagents Final concentration Quantity Unit 123 mM NaN3 (Recipe 5) 6.15 mM 500 μL PBS-Tween (Recipe 1) 9.5 mL Final volume 10 mL Note: No need to adjust pH.
123 mM NaN3
Reagents Final concentration Quantity Unit NaN3 123 mM 400 mg PBS 1× pH 7.4 50 mL Final volume 50 mL Note: No need to adjust pH.
Bleaching solution
Reagents Final concentration Quantity Unit 30% H2O2 6% (1.95 M) 2 mL MetOH 80% (19.75 M) 8 mL Final volume 10 mL Note: Always make a fresh bleaching solution. No need to adjust pH.
25% MetOH
Reagents Final concentration Quantity Unit MetOH 25% (6.17 M) 12.5 mL PBS-Tween (Recipe 1) 75% 37.5 mL Final volume 50 mL Note: No need to adjust pH.
50% MetOH
Reagents Final concentration Quantity Unit MetOH 50% (12.35 M) 25 mL PBS-Tween (Recipe 1) 50% 25 mL Final volume 50 mL Note: No need to adjust pH.
75% MetOH
Reagents Final concentration Quantity Unit MetOH 75% (18.52 M) 37.5 mL PBS-Tween (Recipe 1) 25% 12.5 mL Final volume 50 mL Note: No need to adjust pH.
Blocking solution
Reagents Final concentration Quantity Unit Horse serum 2.5% 250 μL DMSO 5% (0.7 M) 500 μL 123 mM NaN3 (Recipe 5) 6.15 mM 500 μL Roche blocking solution 5% 500 μL Goat serum 10% 1 mL PBS-Tween (Recipe 1) 7.25 Final volume 10 mL Note: No need to adjust pH.
Primary antibody solution
Reagents Final concentration Quantity Unit Monoclonal H3P Ab 1 in 400 (92.5 ng/mL) 10 μL Monoclonal Ecad Ab 1 in 200 (1.25 μg/mL) 20 μL DMSO 5% (0.7 M) 200 μL 123 mM NaN3 (Recipe 5) 6.15 mM 200 μL FBS 10% 400 μL PBS-Tween (Recipe 1) 3.17 mL Final volume 4 mL Note: No need to adjust pH.
Secondary antibody solution
Reagents Final concentration Quantity Unit Polyclonal Anti-Mouse AF555 Ab stock (Recipe 13) 1 in 1,000 (2 μg/mL) 10 μL Polyclonal Anti-Rabbit AF647 Ab stock (Recipe 13) 1 in 800 (2.5 μg/mL) 8 μL DMSO 5% (0.7 M) 200 μL 123 mM NaN3 (Recipe 5) 6.15 mM 200 μL FBS 10% 400 μL PBS-Tween (Recipe 1) 3.182 mL Final volume 4 mL Note: No need to adjust pH.
Secondary antibody stock solution
Reagents Final concentration Quantity Unit Polyclonal secondary Ab 1 in 2 (1 mg/mL) 100 μL Glycerol 50% (6.78 M) 100 μL Final volume 200 μL Note: Mix well. Store at -20 °C. No need to adjust pH.
Nuclear dye solution
Reagents Final concentration Quantity Unit YOYO-1 Iodide 1 in 10,000 (0.1 nM) 1 μL PBS-Tween (Recipe 1) 10 mL Final volume 10 mL Note: The use of cyanine dyes with the iDISCO clearing method is preferred over DAPI (Renier et al., 2014). No need to adjust pH.
DCM-MetOH
Reagents Final concentration Quantity Unit MetOH 33.3% (13 M) 3 mL DCM 66.6% (10.4 M) 6 mL Final volume 9 mL Note: Always handle DCM in chemical hood. Make fresh every time. No need to adjust pH.
DAPI solution
Reagents Final concentration Quantity Unit DAPI 1 mg/mL 2 ng/μL (5.7 μM) 1 μL PBS-Tween (Recipe 1) 499 μL Final volume 500 μL Note: No need to adjust pH.
Phosphate-buffered saline (PBS 1× pH 7.4)
Reagents Final concentration Quantity Unit NaCl 136.89 mM 8 g KCl 2.68 mM 0.2 g Na2HPO4, dibasic, anhydrous 10.14 mM 1.44 g KH2PO4 1.76 mM 0.24 g dH2O 1,000 mL Final volume 1,000 mL Note: Combine ingredients. Adjust pH to 7.4 with 7 N HCl. Bring to final volume with H2O. Autoclave for 20 min.
TrueBlack solution
Reagents Final concentration Quantity Unit TrueBlack lipofuscin 1× 50 µL 200 proof EtOH 665 µL dH2O 285 µL Note: Combine ingredients by vortexing. No need to adjust pH.
Equipment
Shared by both whole mount and cryosections
Open air platform shaker (Eppendorf, New Brunswick, Innova 2000, catalog number: M1190-0000)
Spinning disk confocal microscope (Nikon CSU-W1) or laser scanning confocal microscope (Zeiss, model: LSM 780)
Imaging workstation (65 GB RAM)
Whole mount
Rocking shaker (Fisherbrand, Fisher Scientific, catalog number: 88-861-025)
Pipet controller (Fisherbrand, Fisher Scientific, catalog number: FB14955202)
Cryosections
Cryostat (Leica Microsystems Inc., Leica Biosystems, Leica CM1950)
SlideTray Slide Staining System (Sigma-Aldrich, Z670146-1EA)
Software and datasets
FCS Express 7 Research Edition (De Novo Software, https://denovosoftware.com/)
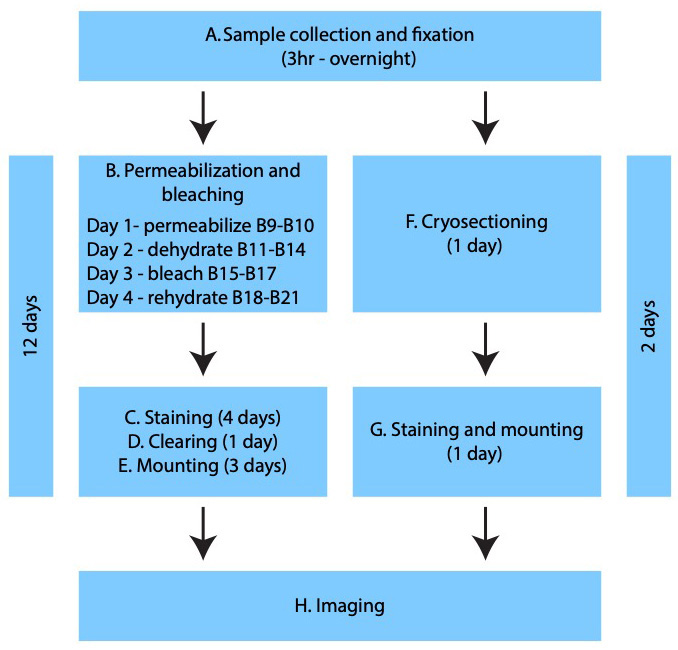
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ortega Granillo, A., Schnittker, R., Wang, W. and Alvarado, A. S. (2023). Quantifying Cell Proliferation Through Immunofluorescence on Whole-Mount and Cryosectioned Regenerating Caudal Fins in African Killifish. Bio-protocol 13(24): e4908. DOI: 10.21769/BioProtoc.4908.
- Wang, W., Hu, C. K., Zeng, A., Alegre, D., Hu, D., Gotting, K., Ortega Granillo, A., Wang, Y., Robb, S., Schnittker, R., et al. (2020). Changes in regeneration-responsive enhancers shape regenerative capacities in vertebrates. Science 369(6508): eaaz3090.
分类
发育生物学 > 细胞生长和命运决定 > 再生
细胞生物学 > 细胞成像 > 胶水印迹
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link