- EN - English
- CN - 中文
An Optimised Indirect ELISA Protocol for Detection and Quantification of Anti-viral Antibodies in Human Plasma or Serum: A Case Study Using SARS-CoV-2
用于检测和定量人血浆或血清中抗病毒抗体的优化间接 ELISA 方案:以 SARS-CoV-2 为例的案例研究
(*contributed equally to this work) 发布: 2023年12月20日第13卷第24期 DOI: 10.21769/BioProtoc.4905 浏览次数: 3552
评审: Alessandro DidonnaHimasha PereraPRASHANT SHARMAChitra Hariharan
Abstract
Advanced immunoassays are crucial in assessing antibody responses, serving immune surveillance goals, characterising immunological responses to evolving viral variants, and guiding subsequent vaccination initiatives. This protocol outlines an indirect ELISA protocol to detect and quantify virus-specific antibodies in plasma or serum after exposure to viral antigens. The assay enables the measurement of IgG, IgA, and IgM antibodies specific to the virus of interest, providing qualitative and quantitative optical densities and concentration data. Although this protocol refers to SARS-CoV-2, its methodology is versatile and can be modified to assess antibody responses for various viral infections and to evaluate vaccine trial outcomes.
Key features
• This protocol builds upon previously described methodology [1] explicitly tailored for SARS-CoV-2 and broadens its applicability to other viral infections.
• The protocol outlines establishing antibody responses to SARS-CoV-2 infections by determining optical densities and concentrations from blood plasma or serum.
Graphical overview
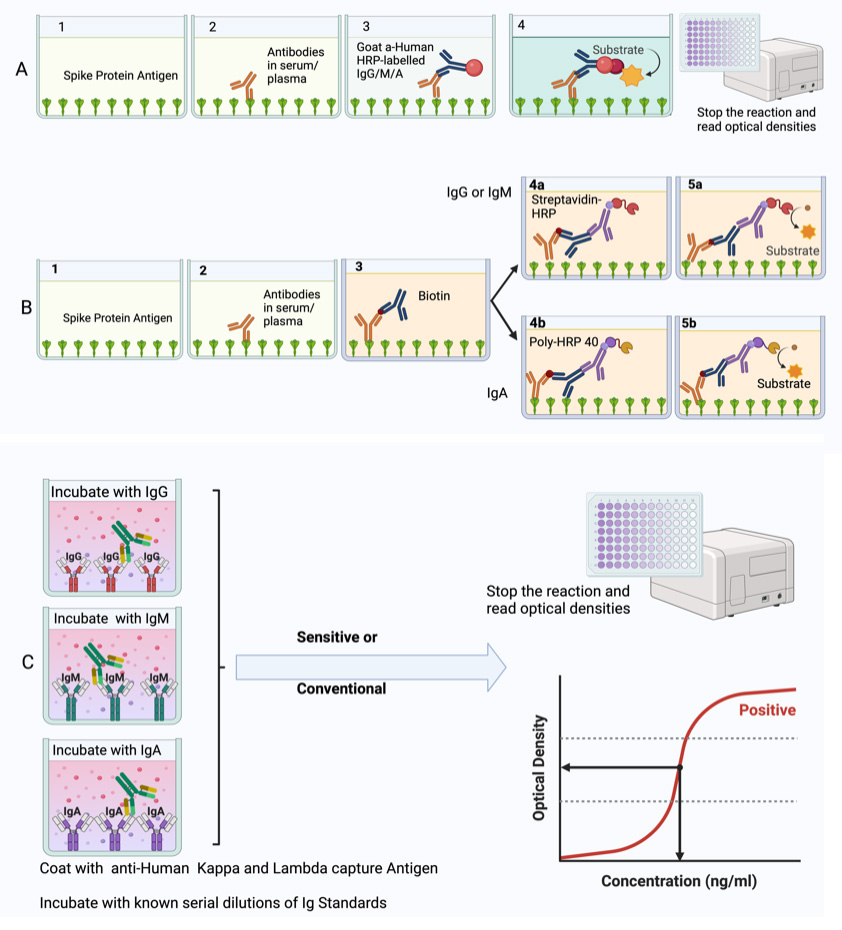
Summary of the conventional ELISA (A) vs. sensitive ELISA (B) procedures. In both A and B, wells are coated with a capture antigen, such as the spike protein, while in (C) they are coated with human Kappa and Lambda capture antibodies. For the conventional ELISA (A), wells with immobilised capture antigens receive serum/plasma containing the target antibody (A1 and B1). This is followed by an HRP-conjugated detection antibody specific to the captured antibody (A2 and B2) and then a substrate solution that reacts with the HRP, producing a colour proportional to the concentration of the antibody in the serum/plasma (A3 and B3). The reaction is halted, and absorbance is measured. In the sensitive ELISA (B), after the serum/plasma addition (A1 and B1), a Biotin-conjugated primary detection antibody is introduced (A2 and B2). Depending on the target antibody, a secondary streptavidin-HRP conjugated detection antibody is added for IgG or IgM (3a) or a poly-HRP 40 detection antibody for IgA (3b). A substrate is introduced, producing a colour change proportional to the antibody concentration (A4 and B4). The reaction is then stopped, and absorbance is measured. In Panel C, wells are coated with human Kappa and Lambda capture antibodies. Serial dilutions of a known antibody standard are introduced. After undergoing the standard ELISA steps, a detection antibody is added, specifically binding to the Ig standard antibody. Subsequently, a substrate solution causes a colour change proportional to the antibody concentration in the serum/plasma. The reaction is halted, and the absorbance of each well is measured. The resulting optical densities from the coated wells form the standard curve, plotting the absorbance against concentrations.
Background
The indirect enzyme-linked immunosorbent assay (ELISA) effectively quantifies and assesses antibody responses, making it invaluable for monitoring public health, evaluating vaccination programs, and testing new vaccines. This has particularly been of importance at the height of the COVID-19 pandemic, during which there was continuous emergence of antigenically distinct variants [2]. It was important to track the dynamics of antigen-specific immune responses to detect immune escape in various populations. Despite similar rates of seropositivity being seen among symptomatic and asymptomatic individuals [3], certain scenarios revealed some differences [4]. A less robust humoral response has been connected to mild COVID-19 cases, prompting concerns about a potentially accelerated decline in immunity. Conversely, severe illness has been linked to a prolonged presence of humoral immunity, enduring for up to 12 months after infection [5, 6]. It is essential to understand the evolution of antibody development within sub-Saharan African (SSA) environments, characterised by distinct disease patterns. Monitoring shifts in IgM, IgG, and IgA levels targeting S, receptor binding domain (RBD), and N antigens becomes crucial in SSA, offering insights into diagnostic approaches, public health strategies, and the immunological aspects relevant to vaccine development. We optimised and validated an in-house indirect ELISA to detect and quantify SARS-CoV-2 spike-, nucleoprotein-, and RBD-directed IgG, IgM, and IgA antibodies in a Ugandan cohort largely characterised by mild and asymptomatic COVID-19. For positive controls, 106 specimens were collected from PCR-confirmed positive study participants; 205 pre-pandemic specimens obtained between October 2012 and November 2017 were used as negative control samples to determine optimal cutoff values, maximizing assay sensitivity and prioritizing assay specificity [1, 7]. Consequently, the determined cutoff values are useful in contexts where mild and asymptomatic SARS-CoV-2 infections are the predominant clinical picture. Commercial standards of known antibody concentrations were used to establish assay limits of detection (LOD) and quantitation (LOQ). Therefore, we present parameters that can be followed to determine optical densities and antibody concentrations in different populations and in regions that had different clinical outcomes and suggest that the same can be done in investigations of antibody responses to other infectious diseases and vaccinations, for example in clinical trials of various vaccine candidates. The COVAC self-amplifying RNA vaccine trial in Uganda [8] has demonstrated the successful application of this protocol.
Materials and reagents
Biological materials
Human serum or plasma samples freshly obtained from study participants, heat inactivated at 56 °C for 30 min and centrifuged at 1,000× g for 10 min at 18–25 °C. One should expect approximately 1.8 mL of serum/plasma after centrifugation, with a pellet at the bottom and the supernatant at the top in each tube. The supernatant should be collected and aliquoted into different tubes stored at -80 °C. Once an aliquot is picked and thawed, it should be kept in a 2–8 °C fridge and used within only one week.
Reagents
Recombinant SARS-CoV-2 Nucleocapsid protein(R&D Systems, catalog number: 10474-CV-01M)
Recombinant SARS-CoV-2 Spike protein (R&D Systems, catalog number: 10549-CV-01M)
Recombinant SARS-CoV-2 Spike S2 Subunit protein (R&D Systems, catalog number: 10594-CV-100)
SARS-CoV-2 RBD (S1) S-protein obtained from a pHL-sec expression vector in HEK 293T/17 cells [obtained from the American Type Culture Collection (ATCC), catalogue number: CRL-11268]. pHL-sec expression vector was a kind donation from the Doores lab at King’s College London
Goat anti-human Kappa-UNLB antibody (Southern Biotech, catalog number: 2060-01)
Goat anti-human Lambda-UNLB antibody (Southern Biotech, catalog number: 2070-01)
Purified human IgG (Sigma, catalog number: I2511)
Purified human IgA (Sigma, catalog number: I2636)
Purified human IgM (Sigma, catalog number: I8260)
Goat anti-human IgG-HRP (Sigma, catalog number: A0170 or AP113P)
Anti-human IgM-HRP (Sigma, catalog number: A6907)
Anti-human IgA-HRP(Sigma, catalog number: A0295)
Goat anti-human Fc IgG-Biotin conjugate (Southern Biotech, catalog number: 2048-08)
Goat anti-human IgA-Biotin (Southern Biotech, catalog number: 2050-08)
Goat anti-human IgM-Biotin (Southern Biotech, catalog number: 2020-08)
PBS/Tween Sachets (PBST) (Sigma, catalog number: P3563)
Tween-20 (Sigma, catalog number: P2287)
1× Dulbecco’s phosphate buffered saline (DPBS), CaCl2 (-) MgCl2 (-) (Sigma, catalog number: D8537)
Bovine serum albumin (BSA) (Sigma, catalog number: A3803)
Goat serum (Sigma, catalog number: G9023)
Streptavidin-HRP (R&D Systems, catalog number: DY988)
Tetramethyl benzidine (TMB) substrate (SeraCare, catalog number: 5120-0080 or 52-00-02)
TMB stop solution (SeraCare, catalog number, 5150-0021 or 50-85-06)
Casein blocking buffer (Thermo Scientific, catalog number: 37582)
Poly-HRP-40 (Fitzgerald, catalog number: 65R-S104PHRP)
Solutions
PBST wash buffer (see Recipes)
BSA assay buffer/sample diluent/blocking buffer (see Recipes)
Goat serum assay buffer/sample diluent (see Recipes)
Recipes
Wash buffer
Reagent Final concentration (%) Quantity or Volume 1× DPBS pH 7.4 99.95% 999.5 mL Tween-20 0.05% 0.5 mL Total 100% 1,000 mL Store in a capped bottle at room temperature up to seven days.
BSA assay buffer/sample diluent/blocking buffer
Reagent Final concentration (%) Quantity or Volume 1× DPBS pH 7.4 99.95% 499.75 mL Tween-20 0.05% 0.25 mL BSA 1% 5 g Total 100% 500 mL Filter the buffer through a 0.22 µm filter unit. Store at 2–8 °C up to seven days.
Goat serum assay buffer/sample diluent
Reagent Final concentration (%) Quantity or Volume 1× DPBS pH 7.4 94% 470 mL Tween-20 1% 5 mL Goat serum 5% 25 mL Total 100% 500 mL Filter the buffer through a 0.22 µm filter unit. Store at 2–8 °C up to seven days.
Laboratory supplies
U-bottom, 96-well dilution plates (Corning, catalog number: 3690)
Medium binding, flat-well ELISA plates (Greiner Bio-One, catalog number: 655001)
Sealing tape (Thermo Fisher Scientific, catalog number: 15036)
Reservoirs (CoStar, catalog number: 4870)
Pipettes (P2, P5, P20, P100, P200, P300-Multichannel, P1000) (at the discretion of the laboratory)
Pipette tips (10, 20, and 200 µL) (at the discretion of the laboratory)
50 mL centrifuge tubes (Sigma, catalog number: T1943)
Paper towel rolls (at the discretion of the laboratory)
2 mL cryovials (Sigma, catalog number: BR114832-1000EA)
0.22 µm syringe filters (TISCH Scientific, catalog number: SPEC16251)
Equipment
-20 °C or -80 °C freezer (at the discretion of the laboratory)
Benchtop centrifuge (at the discretion of the laboratory)
Plate washer (BioTek Washer, ELx405 UCWVSD)
Plate reader (BioTek Reader, Elx808IU)
Software and datasets
Plate reader software: Gen5TM Microplate Data Collection and Analysis Software (v3.12, 11/23/2021); license CD purchased through BioTek or provided along with the reader
Spreadsheet software: Microsoft Office Excel v16.0, 2016 or later (09/22/2015); requires Microsoft Office license.
Data analysis software: GraphPad Prism v9.3 (GraphPad, 11/15/2021); license obtained through GraphPad
Software programming language; R Version 4.3.1 (21/04/2023); free to use
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Baine, C., Jackson, S., Oluka, G. K., Katende, J. S., Ankunda, V. and Serwanga, J. (2023). An Optimised Indirect ELISA Protocol for Detection and Quantification of Anti-viral Antibodies in Human Plasma or Serum: A Case Study Using SARS-CoV-2. Bio-protocol 13(24): e4905. DOI: 10.21769/BioProtoc.4905.
分类
免疫学 > 抗体分析 > 抗体检测
微生物学 > 微生物-宿主相互作用 > 病毒
生物化学 > 蛋白质 > 免疫检测 > 酶联免疫吸附试验(ELISA)
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













