- EN - English
- CN - 中文
Correlative Light and Electron Cryo-Microscopy Workflow Combining Micropatterning, Ice Shield, and an In-Chamber Fluorescence Light Microscope
结合微图案、冰屏蔽和室内荧光显微镜的相关光和电子冷冻显微镜工作流程
(§ Technical contact) 发布: 2023年12月20日第13卷第24期 DOI: 10.21769/BioProtoc.4901 浏览次数: 4412
评审: Abhilash PadavannilLeeya EngelBeatrice LiVamseedhar Rayaprolu
Abstract
In situ cryo-electron tomography (cryo-ET) is the most current, state-of-the-art technique to study cell machinery in its hydrated near-native state. The method provides ultrastructural details at sub-nanometer resolution for many components within the cellular context. Making use of recent advances in sample preparation techniques and combining this method with correlative light and electron microscopy (CLEM) approaches have enabled targeted molecular visualization. Nevertheless, the implementation has also added to the complexity of the workflow and introduced new obstacles in the way of streamlining and achieving high throughput, sample yield, and sample quality. Here, we report a detailed protocol by combining multiple newly available technologies to establish an integrated, high-throughput, optimized, and streamlined cryo-CLEM workflow for improved sample yield.
Key features
• PRIMO micropatterning allows precise cell positioning and maximum number of cell targets amenable to thinning with cryo focused-ion-beam–scanning electron microscopy.
• CERES ice shield ensures that the lamellae remain free of ice contamination during the batch milling process.
• METEOR in-chamber fluorescence microscope facilitates the targeted cryo focused-ion-beam (cryo FIB) milling of these targets.
• Combining the three technologies into one cryo-CLEM workflow maximizes sample yield, throughput, and efficiency.
Graphical overview
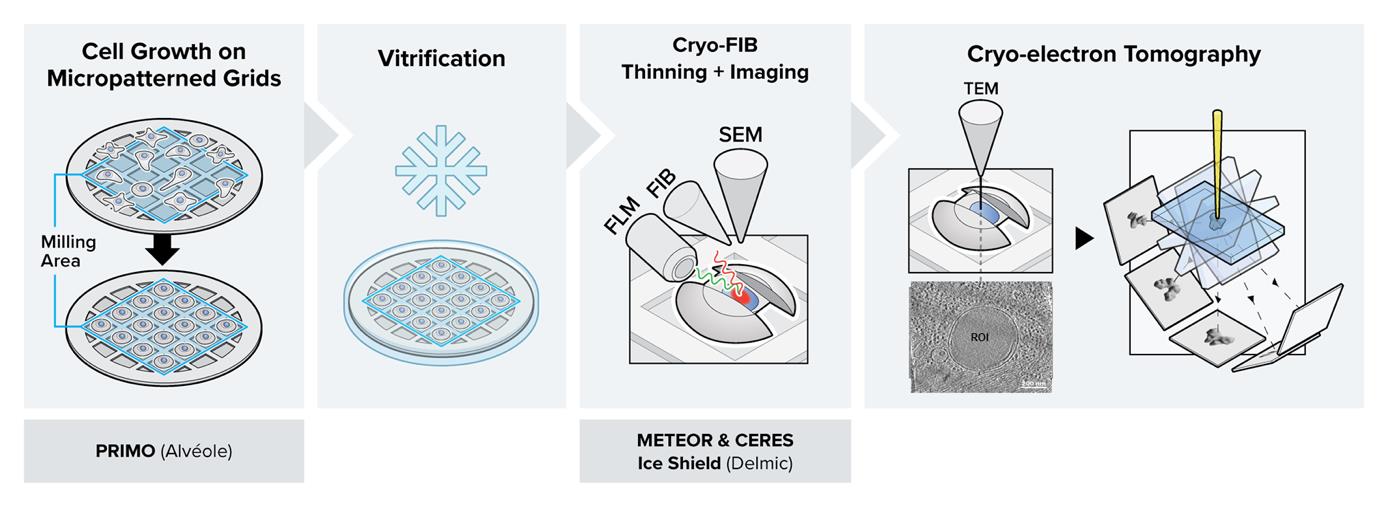
Background
In the conventional in-situ cryo-electron tomography (cryo-ET) workflow, initially cultured cells expressing a fluorescent marker gene are required to adhere to an electron microscopy grid before they are cryo-fixed by plunge freezing (Iancu et al., 2007; Rigort et al., 2012; Mahamid et al., 2016). To navigate through the complex environment of the frozen-hydrated cell, correlative light and electron microscopy (CLEM) approaches were introduced into the workflow to localize the regions of interest (ROIs) (van Driel et al., 2009; Schorb and Briggs, 2014; Klein, Wachsmuth-Melm, et al., 2021). To this end, the grids are typically transferred to a cryo-fluorescence microscope to acquire spatial information on various features of interest using fluorescence (Arnold et al., 2016; Chakraborty et al., 2020). Following a second cryo-transfer step to a cryo focused-ion-beam–scanning electron microscopy (cryo FIB–SEM), the cells are thinned down to ~150 nm-thick lamellae using a focused ion beam, making them amenable to cryo-ET or cryo-EM imaging (Marko et al., 2007; Rigort et al., 2012; Villa et al., 2013; Lucas et al. 2021).
The workflow provides natively preserved insight into the structure-dependent functionality of cellular components at molecular or even close-to-atomic resolution (Xing et al., 2023). However, the complexity of the workflow poses challenges that frequently compromise the throughput and success rate (Hylton and Swulius, 2021). To name a few: i) conventional cell culture procedures lack proper control over cell shape and cell positioning on the grids. This limitation often results in cell clustering and cells positioned on grid bars rather than in the center of the grid squares, both of which make them unsuitable for FIB milling (Wagner et al., 2020). ii) The time-consuming nature of cryo FIB milling requires long residence time of the lamellae within the vacuum chamber. Consequently, the risk of in-chamber ice contamination significantly increases during batch milling sessions, making it impossible to mill multiple lamellae in an overnight session (Buckley et al., 2020; Tacke et al., 2021). iii) Combining CLEM approaches with cryo FIB milling allows for targeted preparation of lamellae at each intended region of interest (ROI). However, the cryo-transfer steps to and from the cryo-fluorescence microscope significantly increase the chance of ice contamination and devitrification of the sample. iv) The conventional way of lamella preparation and target determination is time consuming and complicated. Therefore, the error-prone procedure prior to lamella milling can easily lead to a mismatch in correlation and result in the loss of the ROI (Fukuda et al., 2014; Klein, Wimmer, et al., 2021).
In this protocol, we describe the details of a combined workflow with several newly available technological upgrades. i) Micropatterning the electron microscopy (EM) grids prior to cell culture using PRIMO: the customized patterns result in improved control over cell shape and spatial distribution over the entire grid (Engel et al., 2019; Swistak et al., 2021). By covering the EM grid in an anti-fouling layer and selectively removing it through UV light, the cell can only grow on selected areas of the grid. Provided the applied mask of the UV light is not too big, the cell will adapt to the mask shape, allowing one to reposition neuronal axons, study stress fibers in a specific area of the cell periphery, or adjust the cell shape in some other way. Consequently, more millable sites will be available per grid, making an overnight milling session worthwhile (Toro-Nahuelpan et al., 2020; Sibert et al., 2021). ii) Cryo FIB milling in the presence of the CERES ice shield: the CERES ice shield eliminates the parasitic growth of an amorphous ice layer on top of the lamellae. Without it, lamella can be rendered unusable due to excessive ice contamination at the end of an overnight batch milling session (Tacke et al., 2021; Lau et al., 2022). iii) Continuous, in-chamber fluorescence detection using METEOR: by integrating the METEOR cryo-fluorescence microscope into the vacuum chamber of the cryo FIB–SEM, one major cryo-transfer step is eliminated from the workflow, which significantly reduces the chances of sample devitrification, contamination, and mishandling (Smeets et al., 2021). iv) The fluorescence integration in the FIB–SEM further allows for continuous monitoring of the ROI within the lamella during the milling process, thus eliminating the need for separate signal correlation to find back the ROI. This protocol has been proven to substantially increase the number of produced usable lamellae per milling session and hence surpass the conventional achievable success rate and throughput for high-resolution imaging in a cryo-transmission electron microscope (cryo-TEM). One can normally expect to produce 3–7 lamella in a fully manual milling session, and an estimated 25 lamella in automated session using micropatterned grids and the technological improvements described in this protocol, which are summarized in Table 1.
Table 1. Estimation of the effect of different workflow improvements on the output
| Estimated number of amenable milling sites per grid | Estimated number of final lamellae per grid | |
| Manual milling, no micropatterning | 8 | ~4 |
| Manual milling + micropatterning | 40 | ~8 |
| Manual milling + micropatterning + CERES ice shield | 40 | ~10 |
| Automated milling + micropatterning + CERES ice shield + METEOR | 40 | ~25 |
Materials and reagents
Biological materials
Human immortalized RPE-1 cells (ATCC, hTERT RPE-1, CRL-4000), stably expressing human mCherry-p62
Reagents
200 mesh gold Quantifoil grids R2/1 with SiO2 support layer (Quantifoil, catalog number: N1-S15nAu20-01, 100/pk)
PLL-g-PEG [SuSoS, catalog name: PLL(20)-g[3.5]- PEG(5)]
Poly L-Lysine (Sigma-Aldrich, catalog number: P8920)
mPEG-Succinimidyl Valerate MW 5,000 (mPEG-SVA) (Laysan Bio, Inc. catalog number MPEG-SVA-5000)
14.5 mg/mL PLPP (1 mL PLPP solution) [Alvéole, catalog number: PLPP (for liquid) or PLPP_Gel (for gel)]
Fibronectin (human native fibronectin) (Gibco, catalog number: PHE0023)
Trypan blue (0.4% trypan blue stain) (Invitrogen, catalog number: T10282)
Trypsin (0.05% Trypsin-EDTA solution with phenol red) (Gibco, catalog number: 25300054)
Pen/strep (penicillin-streptomycin, 10.000 U/mL) (Gibco, catalog number: 15140122)
FCS (Fetal Bovine Serum) (Sigma-Aldrich, catalog number: F7524)
Phosphate buffered saline (PBS), pH 7.4 (Gibco, catalog number: 10010056)
DMEM (DMEM/F-12, GlutaMAX supplement) (Gibco, catalog number: 31331028)
Fluorobrite (FluoroBrite DMEM) (Gibco, catalog number: A1896701)
Laboratory supplies
Tweezers (Biological Tweezers, High Alloy DX Style 5) (Electron Microscopy Sciences, catalog number: 78325-5DX)
Parafilm (Parafilm M) (Sigma-Aldrich, catalog number: P7793)
Petri dish (900 mm Petri dish) (VWR, catalog number: 391-2016)
Primo stencil (PDMS stencil with 4 wells) (Alvéole, catalog number: PDMS_STENCILS_4)
30 mm glass-bottom dishes (Cellview cell culture dishes, 35/10 mm, glass bottom) (Greiner Bio-One, catalog number: 627860)
T75 tissue culture flasks (T75 tissue culture flasks) (TPP, catalog number: Z707503-100EA)
Fluorescent marker (Staedtler, Textsurfer classic 364)
Black marker (black laboratory marker) (SecurLine, catalog number: 3083.2)
Cell counting slides (Countess Cell Counting Chamber Slides) (Invitrogen, catalog number: C10228)
Cell strainer (PluriStrainer mini 40 μm) (PluriSelect, catalog number: 43-10040-40)
15 mL falcon tubes (15 mL conical centrifuge tubes) (Falcon, catalog number: 352196)
Eppendorf tubes (Safe-Lock 1.5 mL micro test tubes) (Eppendorf, catalog number: EP0030123328)
1 μm FluoSpheres (FluoSpheres, carboxylate-modified microspheres) (Invitrogen, catalog number: F8816)
Ethane (Air Liquide, catalog number: P0500S10R0A001) or ethane/propane mix (37% ethane/63% propane, Nippon gas, catalog number: 611961)
Grid box for unclipped grids (Jena Biosciences, catalog number: X-CEM-201)
Grid box for clipped grids (Autogrid Compatible Cryo Grid Box) (MiTeGen, catalog number: M-CEM-7AGB)
Autogrid rings with cutout for FIB–SEM (Thermo Fisher Scientific, catalog number: 1205101)
C-ring (Thermo Fisher Scientific, catalog number: 103171)
Equipment
Pelco easiGlow (Pelco easiGLOW Glow discharge Unit) (Ted Pella Inc. catalog number: 91000)
Pelco TEM grid holder block (Ted Pella inc., catalog number: 16820-25)
Fridge (Samsung, model: RL30J3005SA)
Inverted confocal or epifluorescence microscope (Nikon Eclipse Ti2 is used in this protocol)
PRIMO photopatterning device [Alvéole, Photopatterning Device consisting of a (DMD + laser 375 nm, 75 mW) Modulus, Driving Electronics, IHM software LEONARDO]
Mammalian cell incubator (Binder, catalog number: 9040-0112)
Laminar flow hood (Lamsystems, Uniflow, catalog number: 2E-B.003-15)
Water Bath (Julaba Pura 10, catalog number: 9 550 510)
Cell counter (Countess II automated cell counter) (Invitrogen, catalog number: AMQAX1000)
Widefield microscope (Zeiss Axio Vert.A1 FL)
Centrifuge (Eppendorf, model: 5702RH, catalog number: 5704000010)
Vitrobot Mark IV (Thermo Fisher Scientific)
Heating plate (CultureTemp 37 °C, Bel-art Products, catalog number: CHC7.1)
Clipping station (Thermo Fisher Scientific, catalog number: 1130697 or SubAngstrom product code CSA01)
Aquilos2 (Thermo Fisher Scientific)
CERES ice shield (Delmic B.V., catalog number: 2708-999-0010-1)
METEOR system (Delmic B.V., catalog number: 2707-999-0014-2)
High-end cryo TEM with direct electron detector for tomogram acquisition. Here, a 200 kV Talos Arctica G2 (Thermo Fisher Scientific) equipped with a Bioquantum GIF (Gatan) and K3 direct election detector (Gatan) was used
Software and datasets
Inkscape or Adobe Illustrator (2020, version 24.1 used here)
Leonardo (version 4.15, https://www.alveolelab.com/our-products/leonardo-photopatterning-software/) (Access date, September 2023)
xT Microscope Control (version 20.1.1) (Access date, September 2023)
MAPS (version 3.19, https://www.thermofisher.com/order/catalog/product/de/en/MAPS2) (Access date, September 2023)
AutoTEM (version 2.4.1, https://www.thermofisher.com/order/catalog/product/AUTOTEM5?SID=srch-srp-AUTOTEM5) (Access date, September 2023
ODEMIS (version 3.2.2, https://github.com/delmic/odemis) (Access date, September 2023)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Berkamp, S., Daviran, D., Smeets, M., Caignard, A., jani, R., Sundermeyer, P., Jonker, C., Gerlach, S., Hoffmann, B., Lau, K. and Sachse, C. (2023). Correlative Light and Electron Cryo-Microscopy Workflow Combining Micropatterning, Ice Shield, and an In-Chamber Fluorescence Light Microscope. Bio-protocol 13(24): e4901. DOI: 10.21769/BioProtoc.4901.
分类
生物物理学 > 电子冷冻断层扫描
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link