- EN - English
- CN - 中文
Targeted Delivery of Chemogenetic Adeno-Associated Viral Vectors to Cortical Sulcus Regions in Macaque Monkeys by Handheld Injections
通过手持式注射将化学遗传学腺相关病毒载体靶向递送至猕猴皮质沟区域
(*contributed equally to this work, § Technical contact) 发布: 2023年12月05日第13卷第23期 DOI: 10.21769/BioProtoc.4897 浏览次数: 1675
评审: Olga KopachRachael E. HokensonLei GaoVolodymyr Krotov
Abstract
Recent advancements in chemogenetic tools, such as designer receptors exclusively activated by designer drugs (DREADDs), allow the simultaneous manipulation of activity over a specific, broad brain region in nonhuman primates. However, the introduction of DREADDs into large and complexly shaped cortical sulcus regions of macaque monkeys is technically demanding; previously reported methods are time consuming or do not allow the spatial range of expression to be controlled. In the present report, we describe the procedure for an adeno-associated viral vector (AAV2.1) delivery via handheld injections into the dorsolateral prefrontal cortex (Brodmann’s area 9/46) of macaque monkeys, with reference to pre-scanned anatomical magnetic resonance images. This procedure allows the precise delivery of DREADDs to a specific cortical region.
Key features
• This article describes the procedures for injecting viral vectors encoding functional proteins for chemogenetic manipulation into targeted cortical sulcus regions.
• The protocol requires magnetic resonance imaging for the accurate estimation of the injection sites prior to surgery.
• Viral vector solutions are injected using a handheld syringe under microscopic guidance.
• This protocol allows for the precise introduction of designer receptors exclusively activated by designer drugs (DREADDs) to large and complex cortical regions.
Keywords: Monkeys (猴)Background
Genetic approaches that enable the expression of desired functional proteins in order to manipulate the activity of specific brain regions or cell types have become a central method in systems neuroscience [1, 2]. In particular, chemogenetic tools such as designer receptors exclusively activated by designer drugs (DREADDs) have become a promising means to study the relationship between animal behavior and the neuronal activity of specific cell populations. DREADDs allow the manipulation of activity in specific brain regions in behaving animals following systemic administration of an agonist, without any requirements for special devices or implants [3]. This feature is particularly advantageous for manipulating the brain activity of nonhuman primates such as macaque monkeys, which have relatively large and complexly shaped brains.
The introduction of functional proteins to an intended brain region is typically achieved by the injection of viral vector solutions. To manipulate the neuronal activity of a specific region, these vectors need to be precisely delivered over the entire target region. However, if the target region is large and deep within the brain, such as for the cortical sulcus regions, it is technically demanding to inject viral vectors to cover the entire target region. For example, the introduction of DREADDs into a cortical region surrounding a sulcus [e.g., the dorsolateral prefrontal cortex (dlPFC) or Brodmann’s area 46] requires injection of vectors into a broad area along the banks of the principal sulcus (i.e., from the bottom to the surface; Figure 1). A conventional and typical stereotaxic approach using a manipulator ensures accurate localization of viral delivery. However, completing one injection with this procedure takes several tens of minutes, which makes the total surgical duration very long, with considerable surgical stress to the animal. An alternative approach termed convection-enhanced delivery enables widespread cortical viral delivery via a single injection, which considerably shortens the procedural time and minimizes damage [4]. However, its extent of spatial delivery is uncontrollable, which leads to concerns about off-target expression.
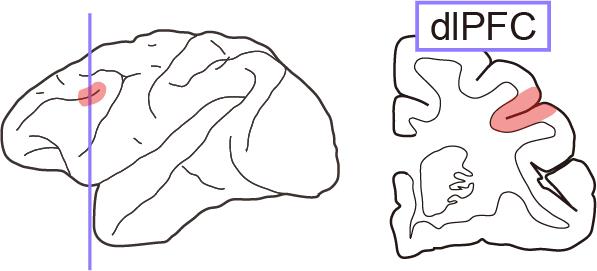
Figure 1. Illustration of the dorsolateral prefrontal cortex (dlPFC). Left, lateral view of the monkey brain. Right, coronal section around the dlPFC (colored area).
In the present report, we describe a procedure for viral vector injection into deep sulcus regions (such as the dlPFC) of macaque monkeys using a handheld injection technique. This procedure enables flexible injection of vectors within 1 min per injection track, each of which has several injection points. This procedure is based on a method described in previous reports [5, 6]. The injections cover 5–10 mm horizontally around the principal sulcus, as we have previously reported [7–9]. Our protocol uses a magnetic resonance (MR) image obtained before surgery to identify the position of the intended injection area, injection tracks and points, and angles for each track. The successful expression of the DREADDs was validated using conventional histological methods.
Materials and reagents
Biological materials
Macaque monkey (5.7 kg; age = 6.0 years old at the beginning of experiments; provided by the National Bio-Resource Project “Japanese Monkeys” of the Ministry of Education, Culture, Sports, Science and Technology, Japan)
Adeno-associated viral vector (e.g., AAV2.1-hSyn-hM4Di-IRES-AcGFP, provided by the Takada Lab, Kyoto University, Japan)
Reagents
Ketamine hydrochloride (Daiichi Sankyo Chemical Pharma, product name: Ketalar for intramuscular injection 500 mg)
Xylazine hydrochloride (Bayer, product name: Seraktar or Rompun)
Sodium cefmetazole (Alfresa Pharma, product name: Cefmetazon for intramuscular injection 0.5 g)
Ketoprofen (Kissei Pharmaceutical, product name: Capisten for intramuscular injection 50 mg)
Lidocaine 2% solution including epinephrine (Sandoz K.K., product name: Xylocaine injection 2% with epinephrine)
Lidocaine spray (Sandoz K.K., product name: Xylocaine pump spray 8%)
Sterilized saline (Otsuka Pharmaceutical, catalog number: 1326)
Povidone-iodine solution (Meiji Seika Pharma, product name: Povidone-iodine 10%)
Propofol (Nichi-Iko, product name: Propofol 1% intravenous injection)
Mannitol (Yoshindo, product name: Mannitol-s injection)
Atropine sulfate (Nipro, product name: Atropine sulfate hydrate 0.5 mg/mL)
Isoflurane [Mylan, product name: Isoflurane inhalation solution (Pfizer)]
Infusion solution (Terumo, catalog number: TP-AB05NR)
Bone wax (TOKYO M.I., catalog number: J901)
Sodium lactate Ringer’s solution (Terumo, product name: Solulact infusion)
Sterilized distilled water (Otsuka Pharmaceutical, catalog number: 1323)
Laboratory supplies
Endotracheal tube (Fuji Systems, catalog number: FR-26)
IV catheter (Terumo, catalog number: SR-FF2419)
25G needle (Terumo, catalog number: NN-2525R)
Surgical tape (Nichiban, catalog number: 21N)
Surgical cotton (Hakujuji, catalog number: 11371)
Disposable electrode for electrocardiogram (Nihon Kohden, catalog number: M-150)
1 mL syringe (Terumo, catalog number: SS-01T)
50 mL syringe (Terumo, catalog number: 5SS-50ESZ)
Valve syringe (Eastsidemed, catalog number: ES-17005)
Micro syringe (Hamilton, model: 1701RN directly connected to a 2 inch PT3 type 30G needle)
Depilatory cream (Center Shoji, product name: Fi-i-mo Epi DX Plus)
Scalpel
No. 11 (Akiyama Medical MFG, catalog number: FS11)
No. 21 (Akiyama Medical MFG, catalog number: FS21)
Suture with needle
Vicryl Plus 3-0 (Ethicon, catalog number: VCP460H)
Vicryl Plus 5-0 (Ethicon, catalog number: VCP303H)
Monosof 2-0 (COVIDIEN, catalog number: SN-628)
Infusion tube (Terumo, catalog number: TK-U200L)
Sterile gown (Nissho Sangyo, catalog number: 24503)
Sterile glove
Overglove (Mölnlycke Health Care, catalog number: 42175)
Underglove (Mölnlycke Health Care, catalog number: 41670)
Sterile drape
1,200 mm × 1,200 mm, no hole (Nissho Sangyo, catalog number: 23203)
900 mm × 900 mm, with hole and tape (Nissho Sangyo, catalog number: 23265)
Ioban antimicrobial incise drape (3M, catalog number: 6661EZ)
Sterile surgical marker (Muranaka Medical Instruments, catalog number: 2730PBX)
Sterile gauze (Hakujuji, catalog number: 14824)
Freer elevator (Roboz, catalog number: RS-8820)
Ruskin rongeur (Biomedical Research Instruments, catalog number: 46-1655)
Knapp scissors (Roboz, catalog number: RS-5965)
Drill bit
Diamond disc, Ø 6–9 mm disc (Biomachinery, custom made)
Twist drill, Ø 2 mm tip (Nakanishi Inc., catalog number: ES-TD-S20)
Equipment
MR imaging scanner (Bruker, model: 7 Tesla 400 mm/SS system)
X-ray computed tomography (CT) scanner (J. Morita, model: 3D Accuitomo170)
Operating microscope (Leica Microsystems GmbH, model: M220)
Patient monitor (Fukuda, model: Bio-Scope AM140)
Shadowless operating lamp (Yamada, model: CRV0404V)
Ventilator (Shin-Ei Industries, Inc., model: A.D.S. 2000)
Vaporizer (Shin-Ei Industries, Inc., model: I-200)
Stereotaxic device (David Kopf Instruments, model: 1530)
Electrolyzed water generator (Omco, model: NDX-70KMW)
Micromanipulator (David Kopf Instruments, model: 2166A)
Micro syringe pump (World Precision Instruments, model: UMP3T-1)
Circulating thermal water system (KIMURAMED, model: T-CARE)
Surgical drill control unit (Nakanishi Inc. model: Primado2)
Software and datasets
PMOD (PMOD Technologies Ltd. ver. 3.6 or above)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Oyama, K., Nagai, Y. and Minamimoto, T. (2023). Targeted Delivery of Chemogenetic Adeno-Associated Viral Vectors to Cortical Sulcus Regions in Macaque Monkeys by Handheld Injections. Bio-protocol 13(23): e4897. DOI: 10.21769/BioProtoc.4897.
分类
神经科学 > 基础技术 > 化学遗传学
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link