- EN - English
- CN - 中文
H2 Production from Methyl Viologen–Dependent Hydrogenase Activity Monitored by Gas Chromatography
通过气相色谱法监测甲基紫精依赖性氢化酶产生氢气的活性
发布: 2023年12月05日第13卷第23期 DOI: 10.21769/BioProtoc.4895 浏览次数: 1754
评审: Chhuttan L MeenaKarem A CourtAnonymous reviewer(s)
Abstract
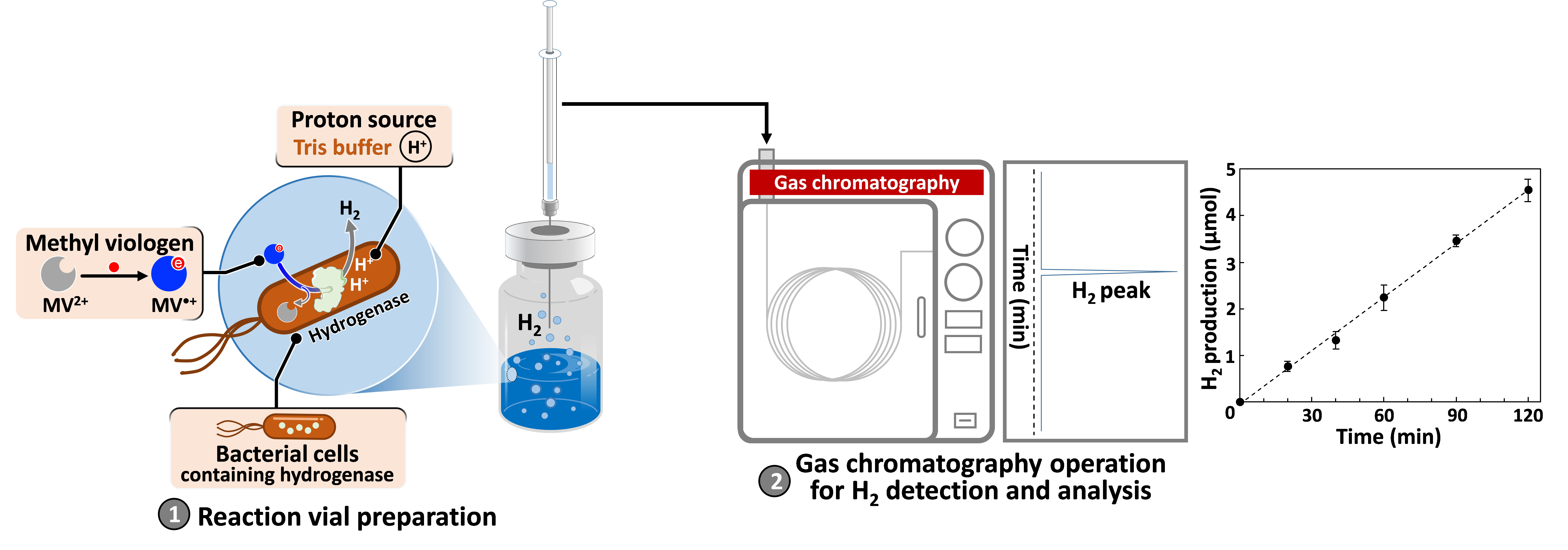
Bio-hydrogen production is an eco-friendly alternative to commercial H2 production, taking advantage of natural systems. Microbial hydrogenases play a main role in biological mechanisms, catalyzing proton reduction to molecular hydrogen (H2) formation under ambient conditions. Direct determination is an important approach to screen bacteria with active hydrogenase and accurately quantify the amount of H2 production. Here, we present a detailed protocol for determining hydrogenase activity based on H2 production using methyl viologen (MV2+) as an artificial reductant, directly monitored by gas chromatography. Recombinant Escherichia coli is used as a hydrogenase-enriched model in this study. Even so, this protocol can be applied to determine hydrogenase activity in all biological samples.
Key features
• This protocol is optimized for a wide variety of biological samples; both purified hydrogenase (in vitro) and intracellular hydrogenase (in vivo) systems.
• Direct, quantitative, and accurate method to detect the amount of H2 by gas chromatography with reproducibility.
• Requires only 2 h to complete and allows testing various conditions simultaneously.
• Kinetic plot of H2 production allows to analyze kinetic parameters and estimate the efficiency of hydrogenase from different organisms.
Graphical overview
Background
Although hydrogen (H2) has been considered as a clean energy carrier, most of today’s commercial H2 production is from fossil fuels, which emit greenhouse gases into the atmosphere. Finding a more sustainable alternative remains a challenge. It is well known that, for over a million years of evolution, microorganisms have acted as a natural H2 production plant through the action of enzymes, namely the hydrogenase family (Tard and Pickett, 2009). With efficient biochemical mechanisms, it is possible to develop an environmentally friendly process for H2 production at ambient conditions by taking advantage of hydrogenase-expressing microorganisms. Among the enzymes with different catalytic sites, [FeFe]-hydrogenase is more efficient than [NiFe]- and [Fe]-types in H2 production with NADH as a reductant in a natural mechanism, as shown in Figure S1 in the Supporting materials (Ogo et al., 2020; Xuan et al., 2023). To screen microorganisms carrying active hydrogenase in a laboratory scale, methyl viologen (MV2+), which is compatible with many enzymes (Orgill et al., 2015), can also be used as an artificial reductant in this reaction. According to the theory, a more negative redox potential of methyl viologen [E(MV2+/MV•+) = -0.446 V vs. normal hydrogen electrode (NHE)] provides a favorable potential scale for proton reduction [E(H+/H2) = -0.41 V vs. NHE] at physiological pH.
In this protocol, a reduced methyl viologen (MV•+) is formed in sodium dithionite (Na2S2O4) solution to serve as an artificial chemical reductant. With cell permeability, MV•+ can penetrate and transfer electrons to intracellular hydrogenase (Kosem et al., 2023), as shown in Figure 1.
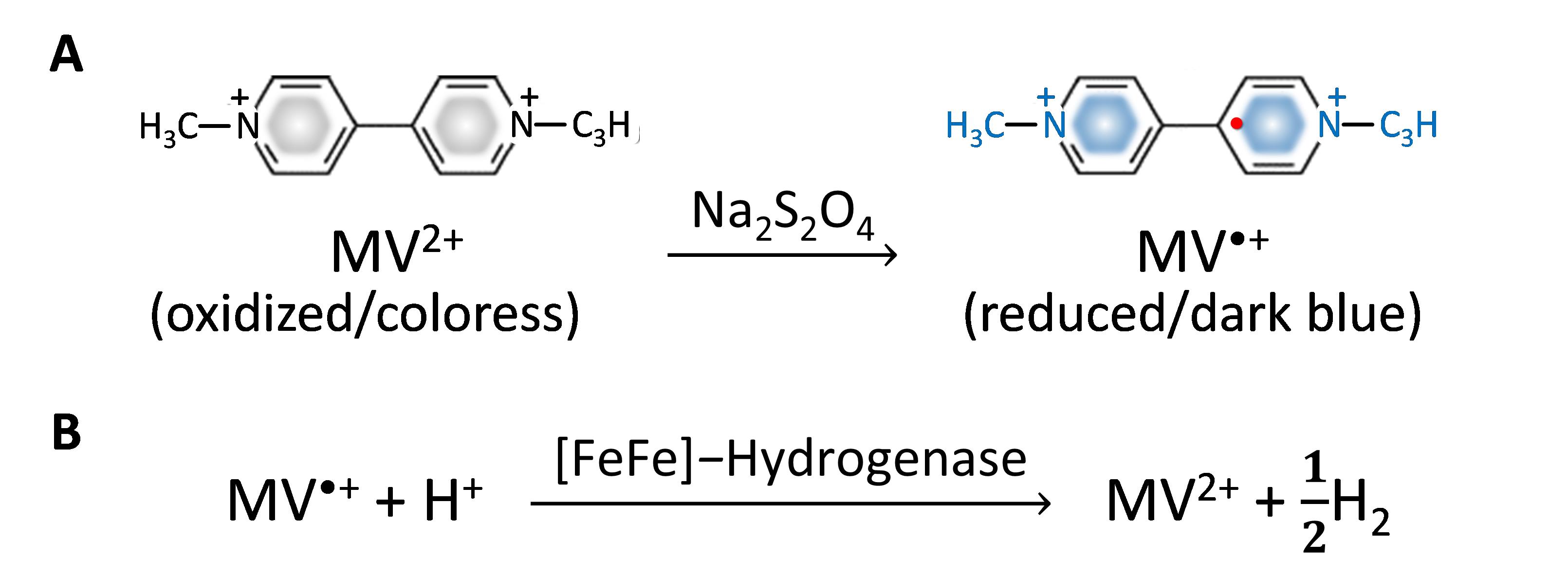
Figure 1. Methyl viologen (MV2+) as an artificial reductant in hydrogenase activity assay. (A) MV2+ reduction to MV•+ formation in Na2S2O4 solution. (B) H2 production from the MV•+-dependent reaction of [FeFe]-hydrogenase.
The amount of H2 generated is directly monitored by gas chromatography. To avoid the detrimental effect of atmospheric oxygen and enhance biocatalytic activity, the reactions are carried out under anaerobic conditions at 37 °C. This method can be applied to determine H2 production capacity of various biological samples such as whole cells, crude cell extracts, or purified enzymes.
Materials and reagents
Biological materials
Hydrogenase-expressing Escherichia coli carrying hydA, hydE, hydF, and hydG genes (this recombinant bacterial strain was constructed in our laboratory according to our previous report in Kosem et al., 2023).
Note: The function of each protein expressed from specific genes in the processes of hydrogenase maturation was reported in many published articles, such as Broderick et al. (2014) and Lubitz et al. (2014).
Reagents
LB broth, Miller (Nacalai Tesque, catalog number: 20068-75)
Methyl viologen (Tokyo Chemical Industry, catalog number: D3685)
Sodium dithionite (Na2S2O4) (Sigma-Aldrich, catalog number: 71699-250G)
PierceTM BCA Protein Assay kit (Thermo Scientific, catalog number: 23227)
Sodium chloride (NaCl) (Wako Chemical, catalog number: 191-01665)
Tris (hydroxymethyl) aminomethane (Tris) (Nacalai Tesque, catalog number: 35434-21)
Hydrochloric acid (HCl) (Wako Chemical, catalog number: 080-0106)
Trichloroacetic acid (TCA) (Nacalai Tesque, catalog number: 34637-14)
3-(N-morpholino)propanesulfonic acid (MOPS) (Nacalai Tesque, catalog number: 23415-25)
Sodium hydroxide (NaOH) (Chameleon Reagent, catalog number: 000-75165)
Ampicillin sodium (Wako, catalog number: 014-23302)
Streptomycin sulfate (Wako, catalog number: 194-08512)
Glucose (Nacalai Tesque, catalog number: 16805-35)
Ferric ammonium citrate (Nacalai Tesque, catalog number: 19425-12)
L-cysteine (Nacalai Tesque, catalog number: 10309-12)
Sodium fumarate (TCI, catalog number: F0070)
Isopropyl-β-D-thiogalactopyranoside (IPTG) (FujiFilm, catalog number: 094-05144)
Solutions
LB (Luria-Bertani) broth (see Recipes)
1 M MOPS-NaOH (see Recipes)
100 mg/mL Ampicillin sodium (see Recipes)
40 mg/mL Streptomycin sulfate (see Recipes)
50% (w/v) Glucose (see Recipes)
250 mg/mL Ferric ammonium citrate (see Recipes)
1 M Sodium fumarate (see Recipes)
1 M IPTG (see Recipes)
0.9% (w/v) NaCl (see Recipes)
50 mM MV2+/Na2S2O4 solution (see Recipes)
1 M Tris-HCl pH 7 (see Recipes)
Recipes
LB broth
Note: Dissolve the medium and all reagents in a 500 mL Erlenmeyer flask and then autoclave at 121 °C for 15 min before use.
Reagent Final concentration Quantity LB broth 2.5 % (w/v) 5 g 1 M MOPS-NaOH (pH 7.4) (Recipe 2) 100 mM 20 mL Water n/a 180 mL Total n/a 200 mL The following (see Recipes 3–8) are added after autoclaving: 100 mg/mL Ampicillin sodium 100 μg/mL 0.2 mL 40 mg/mL Streptomycin sulfate 40 μg/mL 0.2 mL 50% (w/v) Glucose 0.5% (w/v) 2 mL 250 mg/mL Ferric ammonium citrate 250 μg/mL 0.2 mL L-cysteine 2 mM 50 mg 1 M Sodium fumarate 20 mM 4 mL 1 M IPTG 1 mM 0.2 mL 1 M MOPS-NaOH pH 7.4
Note: Dissolve in a 100 mL beaker on a magnetic stirrer.
Reagent Final concentration Quantity MOPS 1 M 20.9 g Water n/a 80 mL NaOH (8 M) n/a Slowly add until pH 7.4 Total n/a Make up final volume to 100 mL 100 mg/mL Ampicillin sodium
Note: Dissolve in a 5 mL microcentrifuge tube and mix thoroughly with gentle shaking by hand. Aliquot the stock solution in 1 mL microcentrifuge tubes (500 μL per tube) and store at -20 °C until use.
Reagent Final concentration Quantity Ampicillin sodium 100 mg/mL 0.5 g Sterilized water n/a 5 mL Total n/a 5 mL 40 mg/mL Streptomycin sulfate
Note: Dissolve in a 5 mL microcentrifuge tube and mix thoroughly with gentle shaking by hand. Aliquot the stock solution in 1 mL microcentrifuge tubes (500 μL per tube) and store at -20 °C until use.
Reagent Final concentration Quantity Streptomycin sulfate 40 mg/mL 0.2 g Sterilized water n/a 5 mL Total n/a 5 mL 50% (w/v) Glucose
Note: Dissolve glucose powder with warm sterilized water in a 100 mL beaker on a magnetic stirrer. Once it has completely dissolved, bring the volume up to 100 mL total. Aliquot the stock solution in 15 mL tubes (10 mL per tube) and store at -20 °C until use.
Reagent Final concentration Quantity Glucose 50% (w/v) 50 g Sterilized water n/a 60 mL Total n/a Make up final volume to 100 mL 250 mg/mL Ferric ammonium citrate
Note: Dissolve in a 5 mL microcentrifuge tube and mix thoroughly with gentle shaking by hand. Aliquot the stock solution in 1 mL microcentrifuge tubes (500 μL per tube) and store at -20 °C until use.
Reagent Final concentration Quantity Ferric ammonium citrate 250 mg/mL 1.25 g Sterilized water n/a 5 mL Total n/a 5 mL 1 M Sodium fumarate
Note: Dissolve in a 50 mL tube and mix thoroughly with gentle shaking by hand. Aliquot the stock solution in 15 mL tubes (10 mL per tube) and store at -20 °C until use.
Reagent Final concentration Quantity Sodium fumarate 1 M 8.0 g Sterilized water n/a 50 mL Total n/a 50 mL 1 M IPTG
Note: Dissolve in a 5 mL microcentrifuge tube and mix thoroughly with gentle shaking by hand. Aliquot the stock solution in 1 mL microcentrifuge tubes (500 μL per tube) and store at -20 °C until use.
Reagent Final concentration Quantity IPTG 1 M 1.2 g Sterilized water n/a 5 mL Total n/a 5 mL 0.9% (w/v) NaCl solution
Note: Dissolve in a 100 mL beaker on a magnetic stirrer.
Reagent Final concentration Quantity NaCl 0.9% (w/v) 0.9 g Water n/a 100 mL Total n/a 100 mL 50 mM MV2+/Na2S2O4 solution
Note: Prepare fresh in an anaerobic sealed vial inside a glove box and mix thoroughly with gentle shaking by hand.
Reagent Final concentration Quantity Methyl viologen 50 mM 0.0257 g Na2S2O4 250 mM 0.0870 g Water n/a 2 mL Total n/a 2 mL 1 M Tris-HCl pH 7
Note: Dissolve in a 100 mL beaker on a magnetic stirrer. Adjust pH with 3 M HCl in a fume hood and strictly following the Kyushu University Guidelines for Safety in Education: Laboratory Activities. A safety data sheet from the chemical company as shown in Guideline 1 in the Supporting materials.
Reagent Final concentration Quantity Tris 1 M 12.1 g Water n/a 80 mL HCl (3 M) n/a Slowly add until pH 7 Total n/a Make up final volume to 100 mL
Laboratory supplies
Glass vial No. 3, 10 mL size (ASONE, catalog number: 5-111-03)
Rubber stopper (ASONE, catalog number: 5-112-01)
Aluminum cap (ASONE, catalog number: 5-112-03)
1 mL Syringe (Terumo, catalog number: SS-01T)
Needle No. 27 G × 3/4" (Terumo, catalog number: NN-2719S)
10 μL pipette tip (Molecular BioProducts, catalog number: 3510-05)
200 μL pipette tip (Violamo, catalog number: 3-6504-12)
1,000 μL pipette tip (Violamo, catalog number: 3-6504-13)
1 mL microcentrifuge tube (Quality Scientific Plastics, catalog number: L-510-GRD-Q)
5 mL microcentrifuge tube (ASONE, model: AST0500)
96-well plate (ASONE, catalog number: 2-8085-02)
99.999% N2 gas (Air Liquide, catalog number: JAGA 13038)
Gloves (Showaglove, catalog number: 882.L.BLUE)
Paper towels
Disposable cuvettes (Violamo, model: UVC-Z8.5)
Equipment
2–20 μL autoclavable micropipette (Nichipet Ex II, Nichiryo, catalog number: J16Y01961)
20–200 μL autoclavable micropipette (Nichipet Ex II, Nichiryo, catalog number: J16Z00871)
100–1,000 μL autoclavable micropipette (Nichipet Ex II, Nichiryo, catalog number: J16X11751)
accu-jet® pro pipette controller (BRAND®, catalog number: Z671533)
High-speed micro centrifuge (Hitachi, model: CF16RN)
Personal centrifuge (Front Lab, model: FLD2012)
Gas chromatography (Shimadzu Corp., model: GC-8A)
Thermal conductivity detector (Shimadzu Corp., model: GC-8AIT)
Integrator C-R6A chromatopac (Shimadzu Corp., catalog number: 223-04500-38)
Molecular sieve 5A beads (GL Sciences Inc., catalog number: 1001-11503)
Stainless steel column, 2 m length × 3 mm diameter (Shimadzu Corp., catalog number: 201-48705-20)
Shaking water bath (Yamato Scientific, model: BW101)
Shaking incubator (EYELA, model: FYC-100)
Anaerobic glove box (MIWA, model: 1ADB-3)
Clean bench (Hitachi Appliances Inc., model: CCV-1300E)
Microplate reader (Corona Electric, model: SH-1000)
500 mL Erlenmeyer flask with baffle (IWAKI, model: CTE33)
250 mL centrifuge bottle (Nalgene, catalog number: B1033)
20 mm vial crimper (Chromatography Research Supplies, catalog number: 320990)
20 mm vial decapper pliers (Kebby, catalog number: D-20)
1 mL gas tight syringe (ITO Corporation, catalog number: MS-GAN100)
Weighing balance with 0.0001 g accuracy (Metter Toledo, model: XS204)
Vortex mixer (Scientific Industries, model: SI-0286)
pH meter (Horiba Scientific, model: 9625)
Water distillation apparatus (Advantec, model: RFD240NA)
Magnetic stirrer (Pasorina Stirrer, model: CT-MINI)
100 mL Beaker (AGC Iwaki, model: CTE33)
Fume hood (Oriental, model: TNV-STZ-1800HCS)
Software and datasets
Microsoft Excel
SF6 (version 5.6.0, Copyright© 2005 Corona Electric) for spectrophotometer
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kosem, N. (2023). H2 Production from Methyl Viologen–Dependent Hydrogenase Activity Monitored by Gas Chromatography. Bio-protocol 13(23): e4895. DOI: 10.21769/BioProtoc.4895.
分类
生物化学 > 蛋白质 > 活性
微生物学 > 微生物生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link