- EN - English
- CN - 中文
Purification of Human Cytoplasmic Actins From Saccharomyces cerevisiae
从酿酒酵母中纯化人类细胞质肌动蛋白
发布: 2023年12月05日第13卷第23期 DOI: 10.21769/BioProtoc.4894 浏览次数: 1743
评审: William Jennings ValentineAlexandros C KokotosAnonymous reviewer(s)
Abstract
Eukaryotic cells rely on actin to support cellular structure, motility, transport, and a wide variety of other cytoplasmic functions and nuclear activities. Humans and other mammals express six closely related isoforms of actin, four of which are found primarily in skeletal, cardiac, and smooth muscle tissues. The final two isoforms, β and γ, are found in non-muscle cells. Due to the ease of purification, many biochemical studies surveying the functions of actin and its regulators have been carried out with protein purified from skeletal muscle. However, it has become increasingly clear that some activities are isoform specific, necessitating more accessible sources of non-muscle actin isoforms. Recent innovations permit the purification of non-muscle actins from human cell culture and heterologous systems, such as insect cell culture and the yeast Pichia pastoris. However, these systems generate mixtures of actin types or require additional steps to remove purification-related tags. We have developed strains of Saccharomyces cerevisiae (budding yeast) that express single untagged isoforms of either human non-muscle actin (β or γ) as their sole actin, allowing the purification of individual homogeneous actin isoforms by conventional purification techniques.
Key features
• Easy growth of yeast as a source of human cytoplasmic actin isoforms.
• Uses well-established actin purification methods.
• The tag-free system requires no post-purification processing.
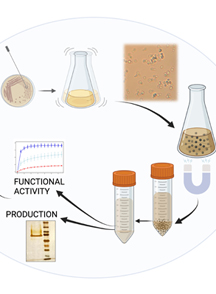
Graphical overview
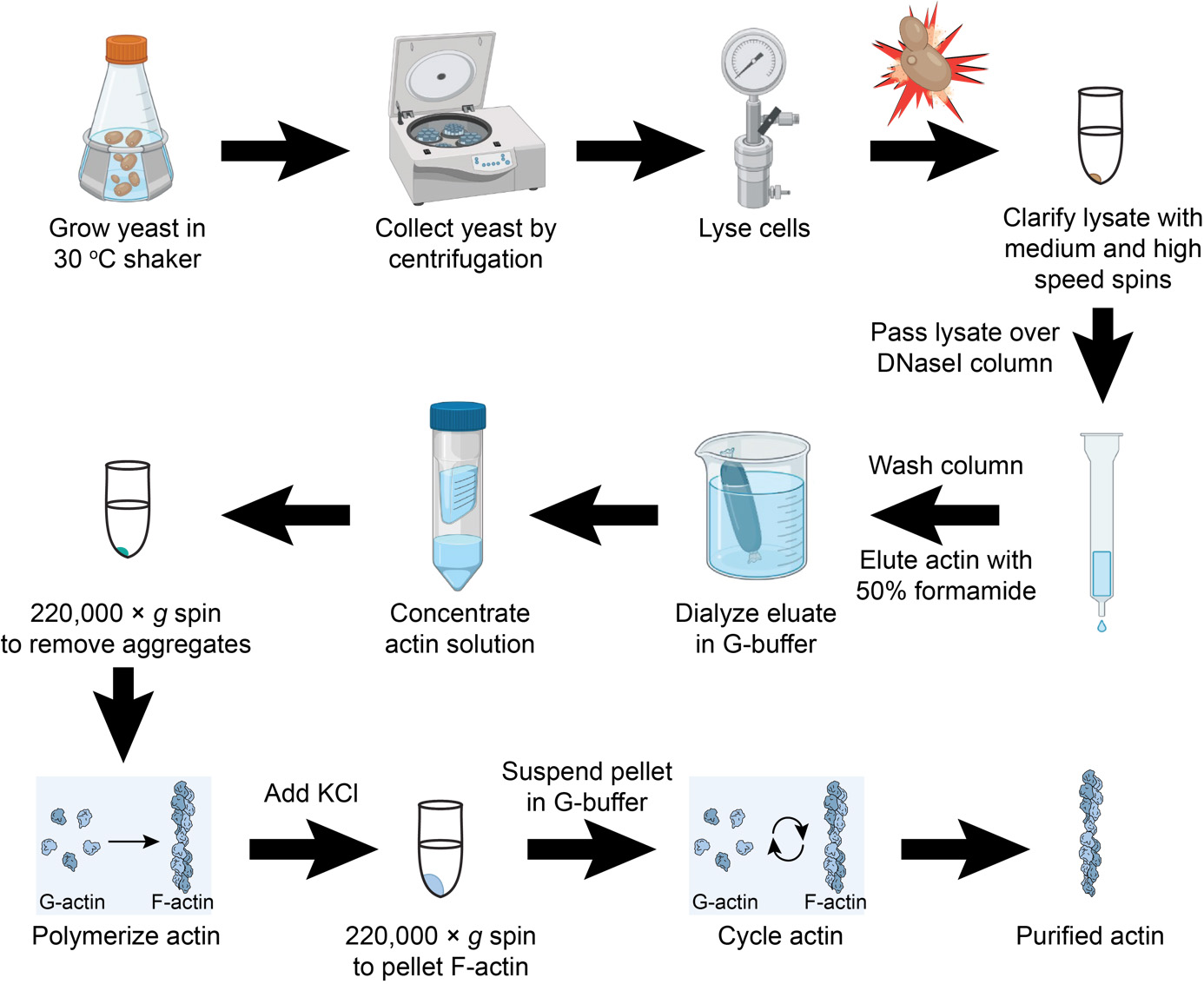
Isolating human cytoplasmic actins from yeast
Keywords: Actin purification (肌动蛋白纯化)Background
Actin and its many regulators are critical to a wide variety of eukaryotic cellular functions, ranging from determination of cell shape and motility to critical nuclear functions that include gene regulation and DNA repair (Belin et al., 2015; Schaks et al., 2019; Kadzik et al., 2020). In humans, the importance of actin regulation is further implicated in cancer metastasis, cardiomyopathies, neurological dysfunctions, and many other debilitating disorders (Rivière et al., 2012; Rubenstein and Wen, 2014; Bamburg and Bernstein, 2016; Hyrskyluoto and Vartiainen, 2020; Izdebska et al., 2020). However, mechanistic advances linking actin to these maladies have been confounded by the presence of six closely related yet distinct actin isoforms. Four actin isoforms are found predominantly in skeletal, cardiac, and smooth muscle tissues (α1, α2, α-cardiac, and γ2), and two are found primarily in non-muscle cells (β and γ1, commonly referred to as cytoplasmic actins). Further, much of what we know about the biochemical nature of critical actin regulators has been deciphered using the readily abundant and easily obtained 1 actin from animal muscle sources (e.g., rabbit, chicken, or cow). While muscle actin has been invaluable for the study of actin organization, regulation, and dynamics, it is becoming increasingly clear that some actin regulators display isoform specificity (De La Cruz, 2005; Perrin and Ervasti, 2010; Antoku et al., 2019). Thus, reliable sources of pure cytoplasmic actin isoforms are required to effectively explore these differing functions. However, unlike the muscle actin isoform α1, these isoforms are difficult to obtain in suitable concentrations and purity for widespread adoption and use.
Initially developed methods obtained mixtures of cytoplasmic actins from human cells (Schafer et al., 1998); similar mixtures of β and γ1 are available from commercial sources (Cytoskeleton, Inc., Denver, CO). Newer systems involving cleavable fusion proteins expressed in human cells or in the yeast Pichia pastoris improved purity to individual isoforms and extended expression to disease-relevant mutations in actin isoforms (Hatano et al., 2018; Hatano et al., 2020; Ceron et al., 2022). These purification systems have the further benefit of extending isoform analysis to important actin post-translational modifications, such as His73 methylation, and N-terminal processing. We produced a different, relatively inexpensive system that yields Human β or γ1 actin from the budding yeast Saccharomyces cerevisiae. Here, we combine engineered yeast strains with the classic purification of actin via DNase I affinity and elution with the denaturant formamide (Zechel et al., 1980; Kilmartin and Adams 1984; Kron et al., 1992; Goode 2002). This provides a relatively simple technique that does not require additional column chromatography. These and related sources of human cytoplasmic actins (Hatano et al., 2018; Hatano et al., 2020; Ceron et al., 2022) will play an important role in understanding the biochemical activities of non-muscle actin regulatory proteins, which may provide important new insights into actin-based disease development and progression.
Materials and reagents
Biological materials
BHY845 [MATa ura3∆0 leu2∆0 his3∆1 act1∆::NatR (pBH839 = 2μ LEU2 GPDpr-HsACTB)] S. cerevisiae strain that expresses only human β actin (Haarer et al., 2023); available with material transfer agreement (MTA) due to pending patent application (see conflict of interest statement).
BHY848 [MATα ura3∆0 leu2∆0 his3∆1 mfa1∆:: MFA1pr-SpHIS5 act1∆::NatR (pBH869 = 2μ LEU2 GPDpr-HsACTG1)] S. cerevisiae strain that expresses only human γ actin (Haarer et al., 2023); available on request, as above.
Reagents
Bacto peptone (BD-Gibco, catalog number: 211677)
Bacto yeast extract (BD-Gibco, catalog number: 212750)
Bacto agar (BD-Difco, catalog number: 214010)
Glucose (Sigma, catalog number: G-7021; Mallinckrodt, catalog number: 4908)
Glacial acetic acid (Fisher, catalog number: A38-212)
Hydrochloric acid (HCl) (Fisher, catalog number: A144S-212)
Tris base (Fisher, BP152-5) or Trizma base (Sigma, catalog number: T6066)
Sodium acetate (J.T. Baker, catalog number: 3470-01; Fisher, catalog number: BP333-500)
Sodium chloride (NaCl) (Sigma, catalog number: S7653)
Sodium bicarbonate (NaHCO3) (Sigma, catalog number: S5761)
Formamide, deionized (Sigma, catalog number: F9037)
Ammonium chloride (NH4Cl) (Sigma, catalog number: A9434)
Potassium chloride (KCl) (J.T. Baker, catalog number: 3040-01)
Calcium chloride dihydrate (CaCl2·2H2O) (Fisher, catalog number: BP510-500)
CNBr-activated Sepharose 4B (Cytiva, catalog number: 17-0430-01)
Deoxyribonuclease I (DNase I) (Worthington, catalog number: LS002007)
Bio-Rad protein assay dye reagent (Bio-Rad, catalog number: 5000006)
Calbiochem protease inhibitor cocktail IV (Millipore-Sigma, catalog number: 539136)
Phenylmethylsulfonyl fluoride (PMSF) (Sigma, catalog number: P7626)
Magnesium chloride, 6-hydrate (MgCl2·6H2O) (J.T. Baker, catalog number: 4003-01)
Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) (Sigma, catalog number: E4378)
Adenosine 5′-triphosphate, disodium trihydrate (ATP) (GoldBio, catalog number: A-081-25)
Dithiothreitol (DTT) (Bio-Rad, catalog number: 161-0611; GoldBio, catalog number: DTT25)
Potassium hydroxide (KOH) (Fisher, catalog number: P250-500)
Sodium hydroxide (NaOH) (Fisher, catalog number: BP359-500)
Sodium azide (Fisher, catalog number: BP922I-500)
Solutions
40% (w/v) glucose
1 mM HCl
1 M Tris pH 8.0
1 M Tris pH 7.5
3 M KCl
1 M MgCl2
0.5 M CaCl2
5 M NaCl
2% sodium azide
YPD medium (1 L), liquid (see Recipes)
YPD medium (1 L), plates (see Recipes)
40% glucose (1 L) (see Recipes)
Coupling buffer (250 mL) (see Recipes)
Low pH wash buffer (100 mL) (see Recipes)
High pH wash buffer (100 mL) (see Recipes)
0.5 M sodium acetate, pH 4 (500 mL) (see Recipes)
1 M tris, pH 7.5 or pH 8.0 (1 L) (see Recipes)
DNase I storage buffer (100 mL) (see Recipes)
G-buffer (50 mL) (see Recipes)
Lysis buffer (50 mL) (see Recipes)
Actin wash buffer 1 (50 mL) (see Recipes)
Actin wash buffer 2 (50 mL) (see Recipes)
Actin elution buffer (20 mL) (see Recipes)
20× F-buffer (1 mL) (see Recipes)
100 mM PMSF (100 mL) (see Recipes)
0.5 M ATP (20 mL) (see Recipes)
1 M DTT (50 mL) (see Recipes)
0.5 M EGTA (100 mL) (see Recipes)
Recipes
YPD medium (1 L), liquid
Reagent Final concentration Quantity Bacto peptone 2% (w/v) 20 g Bacto yeast extract 1% (w/v) 10 g H2O 900 mL Glucose (dextrose) 4% (w/v) 100 mL of 40% stock (see Note*, below) *Note: Autoclave the peptone and yeast extract solution separately from the 40% glucose solution. This limits media caramelization and reaction with media components. Thus, to complete the YPD, add the sterile peptone-yeast solution to the sterile glucose aseptically (i.e., 900 mL of peptone-yeast solution and 100 mL of 40% glucose). Separate media components can be stored for several weeks at room temperature or at 4 °C for longer periods.
YPD medium (1 L), plates
Reagent Final concentration Quantity Bacto peptone 2% (w/v) 20 g Bacto yeast extract 1% (w/v) 10 g Bacto agar 1.8% (w/v) 18 g (see note*) H2O 900 mL Glucose (dextrose) 4% (w/v) 100 mL of 40% stock (see Note*, below) *Note: For plates, add agar to peptone and yeast extract solution, add magnetic stir bar, and autoclave. After autoclaving, add sterile 40% glucose solution and place on stir plate with slow stirring to avoid bubbles; let cool to ~55 °C. Carefully pour media plus agar solution into (35–40) sterile Petri dishes (10 cm) and let sit on bench for 1–2 days at room temperature; plates can be stored upside down in sealed Petri dish bags for several weeks at room temperature or at 4 °C for longer periods.
40% glucose (1 L)
Filter sterilize or autoclave
Reagent Quantity Glucose (dextrose) 400 g H2O to 1 L Coupling buffer (250 mL)
Reagent Final concentration Stock solution Quantity Sodium bicarbonate 0.1 M 2.1 g Sodium chloride 0.5 M 5 M 25 mL Calcium chloride 0.1 mM 0.5 M 50 μL H2O Final volume to 250 mL Low pH wash buffer (100 mL)
Reagent Final concentration Stock solution Quantity Sodium acetate, pH 4 0.1 M 0.5 M, pH 4 20 mL Sodium chloride 0.5 M 5 M 10 mL H2O 70 mL High pH wash buffer (100 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 8 0.1 M 1 M, pH 8 10 mL Sodium chloride 0.5 M 5 M 10 mL H2O 80 mL 0.5 M sodium acetate, pH 4 (500 mL)
Reagent Quantity Sodium acetate 20.5 g Glacial acetic acid to pH 4.0 H2O to 500 mL 1 M tris, pH 7.5 or pH 8.0 (1 L)
Reagent Quantity Tris base 121.1 g HCl to pH 7.5 or 8.0 H2O to 1 L DNase I storage buffer (100 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 50 mM 1 M 5 mL Sodium chloride 0.5 M 5 M 10 mL Sodium azide 0.02% (w/v) 2% (w/v) 1 mL H2O 84 mL G-buffer (50 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.5 mL Calcium chloride 0.2 mM 0.5 M 20 μL ATP 0.5 mM 0.5 M 50 μL (see Note*, below) DTT 0.2 mM 1 M 10 μL* H2O 49.4 mL* *Note: Add ATP and DTT solutions the day of use; store G-buffer with ATP and DTT on ice or at 4 °C.
Lysis buffer (G-buffer and protease inhibitors; 50 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.5 mL Calcium chloride 0.2 mM 0.5 M 20 μL ATP 0.5 mM 0.5 M 50 μL DTT 0.2 mM 1 M 10 μL PMSF 0.1 mM 0.1 M 50 μL Calbiochem protease inhibitor cocktail IV 0.2% (v/v) 100 μL H2O 49.27 mL Actin wash buffer 1 (G-buffer and 10% formamide; 50 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.5 mL Calcium chloride 0.2 mM 0.5 M 20 μL ATP 0.5 mM 0.5 M 50 μL DTT 0.2 mM 1 M 10 μL PMSF 0.1 mM 0.1 M 50 μL Formamide 10% (v/v) 5 mL H2O 44.37 mL Actin wash buffer 2 (G-buffer and 0.2 M NH4Cl; 50 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.5 mL Calcium chloride 0.2 mM 0.5 M 20 μL ATP 0.5 mM 0.5 M 50 μL DTT 0.2 mM 1 M 10 μL Ammonium chloride 0.2 M 0.5 M 20 mL H2O 29.42 mL Actin elution buffer (G-buffer and 50% formamide; 20 mL)
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 0.2 mL Calcium chloride 0.2 mM 0.5 M 8 μL ATP 0.5 mM 0.5 M 20 μL DTT 0.2 mM 1 M 4 μL Formamide 50% (v/v) 10 mL H2O 9.77 mL 20× F-buffer (1 mL)
10 mM tris pH 7.5, 500 mM KCl, 80 mM MgCl2, 20 mM EGTA, 10 mM ATP
Reagent Final concentration Stock solution Quantity Tris, pH 7.5 10 mM 1 M 10 μL Potassium chloride 0.5 M 3 M 167 μL Magnesium chloride 80 mM 1 M 80 μL EGTA 20 mM 0.5 M 40 μL ATP 10 mM 0.5 M 20 μL H2O 683 μL 100 mM PMSF (100 mL)
Store at -20 °C
Reagent Quantity PMSF 1.74 g 100% ethanol to 100 mL 0.5 M ATP (20 mL)
Store filter-sterilized frozen aliquots at -20 °C
Reagent Quantity ATP 6.05 g KOH to pH ~ 7.2 H2O to 20 mL 1 M DTT (50 mL)
Store filter-sterilized frozen aliquots at -20 °C
Reagent Quantity DTT 7.71 g H2O to 50 mL 0.5 M EGTA (100 mL)
Reagent Quantity EGTA
NaOH
19.0 g
to pH 8
H2O to 100 mL
Laboratory supplies
50 mL conical tubes (Thermo Fisher Scientific, Falcon, catalog number: 1495949A)
15 mL conical tubes (Thermo Fisher Scientific, Falcon, catalog number: 1495949B)
Pierce 5 mL disposable columns, including polyethylene discs, stoppers, and end caps (Thermo/Pierce, catalog number: 29922)
Funnels for Pierce columns (Thermo/Pierce, catalog number: 29923)
Petri dishes (Kord-Valmark, catalog number: 2900)
2 L Erlenmeyer flasks
Syringe filters, 0.45 μm (Genesee Scientific, catalog number: 25-242)
Slide-A-Lyzer dialysis cassettes, 7,000 or 10,000 MWCO (Thermo Fisher Scientific, catalog numbers: 66370 and 66380, respectively)
Dialysis tubing, 12,000–14,000 MWCO, 25 mm width (Fisher Scientific, catalog number: 21-152-18)
Protein concentrators: Vivaspin-6, 5,000 MWCO (Sartorius, catalog number: VS0611); Amicon Ultra-4, 10,000 MWCO (Millipore-Sigma, catalog number: UFC801024)
Equipment
Magnetic stir plate (optional; for making YPD plates)
Shaking incubator capable of holding multiple 2 L flasks at 30 °C
Spectrophotometer for cell density measurement (Bio-Rad, model: SmartSpec 3000)
Rocker or rotator, preferably at 4 °C (e.g., in cold room or chromatography cabinet)
Centrifuges:
Refrigerated tabletop centrifuge (Beckman Coulter, model: Allegra X-15R) for preparing resin
Superspeed centrifuge (Thermo Scientific/Sorvall, model: Lynx 4000) and rotor (Thermo Scientific, model: F10-4x1000 LEX) for harvesting 1–2 L of yeast
Ultracentrifuge (Beckman Coulter, model: Optima LE-80K) and rotor (Beckman Coulter, model: 70 Ti) for clarifying yeast lysates
Tabletop ultracentrifuge (Beckman Coulter, model: Optima Max-XP) and rotors (Beckman Coulter, models: TLA100, TLA100.2, TLA100.3) for clarifying actin solutions
French press and pressure cell with 1-inch diameter piston (American Instrument Company/SIM-Aminco)
Protein gel apparatus and power supply (Bio-Rad, models: Mini-Protean Tetra, PowerPac Basic Power Supply)
Optional: spectrophotometer for determining protein concentration (Thermo Scientific, model: NanoDrop 2000)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Haarer, B. K., Amberg, D. C. and Henty-Ridilla, J. L. (2023). Purification of Human Cytoplasmic Actins From Saccharomyces cerevisiae. Bio-protocol 13(23): e4894. DOI: 10.21769/BioProtoc.4894.
分类
微生物学 > 异源表达系统 > 酿酒酵母
生物化学 > 蛋白质 > 分离和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link