- EN - English
- CN - 中文
Reprogramming Cancer Cells to Antigen-presenting Cells
将癌细胞重编程为抗原呈递细胞
发布: 2023年11月20日第13卷第22期 DOI: 10.21769/BioProtoc.4881 浏览次数: 4996
评审: Shalini Low-NamScott McCombDavide Botta
Abstract
Cancer cells evade the immune system by downregulating antigen presentation. Although immune checkpoint inhibitors (ICI) and adoptive T-cell therapies revolutionized cancer treatment, their efficacy relies on the intrinsic immunogenicity of tumor cells and antigen presentation by dendritic cells. Here, we describe a protocol to directly reprogram murine and human cancer cells into tumor-antigen-presenting cells (tumor-APCs), using the type 1 conventional dendritic cell (cDC1) transcription factors PU.1, IRF8, and BATF3 delivered by a lentiviral vector. Tumor-APCs acquire a cDC1 cell-like phenotype, transcriptional and epigenetic programs, and function within nine days (Zimmermannova et al., 2023). Tumor-APCs express the hematopoietic marker CD45 and acquire the antigen presentation complexes MHC class I and II as well as co-stimulatory molecules required for antigen presentation to T cells, but do not express high levels of negative immune checkpoint regulators. Enriched tumor-APCs present antigens to Naïve CD8+ and CD4+ T cells, are targeted by activated cytotoxic T lymphocytes, and elicit anti-tumor responses in vivo. The tumor-APC reprogramming protocol described here provides a simple and robust method to revert tumor evasion mechanisms by increasing antigen presentation in cancer cells. This platform has the potential to prime antigen-specific T-cell expansion, which can be leveraged for developing new cancer vaccines, neoantigen discovery, and expansion of tumor-infiltrating lymphocytes.
Key features
• This protocol describes the generation of antigen-presenting cells from cancer cells by direct reprogramming using lineage-instructive transcription factors of conventional dendritic cells type I.
• Verification of reprogramming efficiency by flow cytometry and functional assessment of tumor-APCs by antigen presentation assays.
Background
Immune evasion, an important hallmark of cancer, is characterized by the exclusion of effector immune cells and immunosuppression, the inherent heterogeneity of cancer cells and reduced antigen presentation, which all contribute to tumor immunogenicity (Sharma et al., 2017; Jhunjhunwala et al., 2021). Immune checkpoint inhibition (ICI) revolutionized cancer treatment by enhancing the patient’s immune system, resulting in long-term responses in melanoma and other types of cancer. Nevertheless, some cancers, such as prostate cancer and glioblastoma, are more resistant to immunotherapeutic approaches. Additionally, among indications eligible for immunotherapy, responses vary greatly across patients (Sharma et al., 2017). Antigen presentation and tumor’s intrinsic immunogenicity play crucial roles in promoting efficient CD8+ and CD4+ T-cell responses during ICI treatment (Manguso et al., 2017; Patel et al., 2017; Oh et al., 2020; Blomberg et al., 2023). Immune evasion mechanisms contribute to tumor progression by reducing immunogenicity through upregulation of immune checkpoints (Hashimoto et al., 2018), tumor antigen editing, and downregulation of antigen presentation pathways (Jhunjhunwala et al., 2021). These mechanisms arise as a consequence of transcriptional and epigenetic alterations in antigen processing and presentation pathways (Guo et al., 2021) and deregulation of interferon (IFN) signaling (Kalbasi and Ribas, 2020). Despite attempts at increasing tumor immunogenicity by manipulating the IFN-γ pathway (Griffin et al., 2021; Guo et al., 2021), successful strategies to engineer tumor immunogenicity are still lacking. For the past decade, conventional dendritic cells type I (cDC1) have been established as key players in anti-tumor immunity. cDC1 cells are specialized in cross-presenting tumor antigens to CD8+ T cells, which is fundamental for successful anti-tumor immunity (Poulin et al., 2012; Barry et al., 2018; Kvedaraite and Ginhoux, 2022). Additionally, cDC1 cells recruit and orchestrate immune effectors through the secretion of pro-inflammatory chemokines and cytokines (Spranger et al., 2017). Importantly, the presence of cDC1 cells within the tumor microenvironment correlates positively with immunotherapy’s success and better survival in humans (Spranger et al., 2017; Hubert et al., 2020; Mayoux et al., 2020).
Recently, a cDC1 cells–based vaccine promoted tumor rejection and abscopal tumor control in murine models, independently of host’s cDC1 cell compartment (Ferris et al., 2022). Current methods to generate cDC1 cells rely on the culture of peripheral blood mononuclear cells (PBMCs), bone marrow (BM) progenitors, or monocytes with cytokine cocktails that promote cDC1 cell differentiation (Poulin et al., 2010; Balan and Dalod, 2016). Efforts have also been placed to differentiate leukemic blasts from acute myeloid leukemia into leukemia-derived dendritic cells with cytokine cocktails (Amberger and Schmetzer, 2020). However, ex vivo production of blood-derived dendritic cells is costly, has limited yields, and the differentiation approach is inefficient and limited to blood cancers (Amberger and Schmetzer, 2020; Makino et al., 2022). On the other hand, direct reprogramming approaches have the advantage to generate the desired cell fate from any cell type, have great potential for in vivo application, and are more suited for clinical applications as transdifferentiation processes often bypass progenitor cell states (Wang et al., 2021; Zimmermannova et al., 2021). Our group has defined the minimum network of transcription factors to induce cDC1 cell’s identity through overexpression of PU.1, IRF8, and BATF3 in fibroblasts (Rosa et al., 2018, 2020 and 2022). While this approach could be potentially used to generate induced cDC1 cells, we have hypothesized that the same combination of transcription factors could restore immunogenicity in cancer cells. Cancer cells retain cellular plasticity that allows the modification of cancer cell fate with direct cellular reprogramming (Hochedlinger et al., 2004; Suva et al., 2013; Ishay-Ronen et al., 2019; Zimmermannova et al., 2021), and we have recently demonstrated that ectopic expression of PU.1, IRF8, and BATF3 induces a cDC1 cell–like state in cancer cells (Zimmermannova et al., 2023). Reprogramming of mouse and human cancer cells to antigen-presenting cells occurs by imposing a cDC1 cells’ transcriptional and epigenetic program. Tumor-antigen-presenting cells (tumor-APCs) acquire a cDC1 cell–like immunophenotype, respond to toll-like receptor (TLR) stimuli to secrete pro-inflammatory cytokines, as well as engulf, process, and cross-present antigens as early as day 3 of reprogramming. Tumor-APCs also show endogenous antigens more promptly, becoming targets for T cell–mediated killing, and upon intratumorally injection reduce tumor growth and increase survival in mice (Zimmermannova et al., 2023).
Here, we describe a protocol to generate tumor-APCs within nine days and follow the process at the phenotypic and transcriptional levels. We also describe a magnetic-activated cell sorting (MACS) protocol to purify a high number of tumor-APCs that can be used for in vitro and in vivo assays. While fluorescence-activated cell sorting (FACS) is used to purify rare populations with higher purity, MACS-based purification recovers more viable cells in 4–6-fold less time than FACS (Sutermaster and Darling, 2019; Pan and Wan, 2020). Additionally, MACS requires less expensive instrumentation than FACS, and is easily scalable without adding significantly more processing time. MACS purification of tumor-APCs recovers an average of 2 × 106 of tumor-APCs per 1 × 106 of original cells seeded at day -1 (Figure 1). We also illustrate the functionality of MACS-purified tumor-APCs by performing antigen presentation assays to Naïve CD4+ T cells. As tumor-APCs can be generated from different cancer types, including mouse and human cell lines and primary tumor cells (Zimmermannova et al., 2023), this technology supports a vast array of in vitro approaches that require large numbers of tumor-APCs, e.g., in vitro tumor antigen-specific T-cell expansion or neoantigen identification.
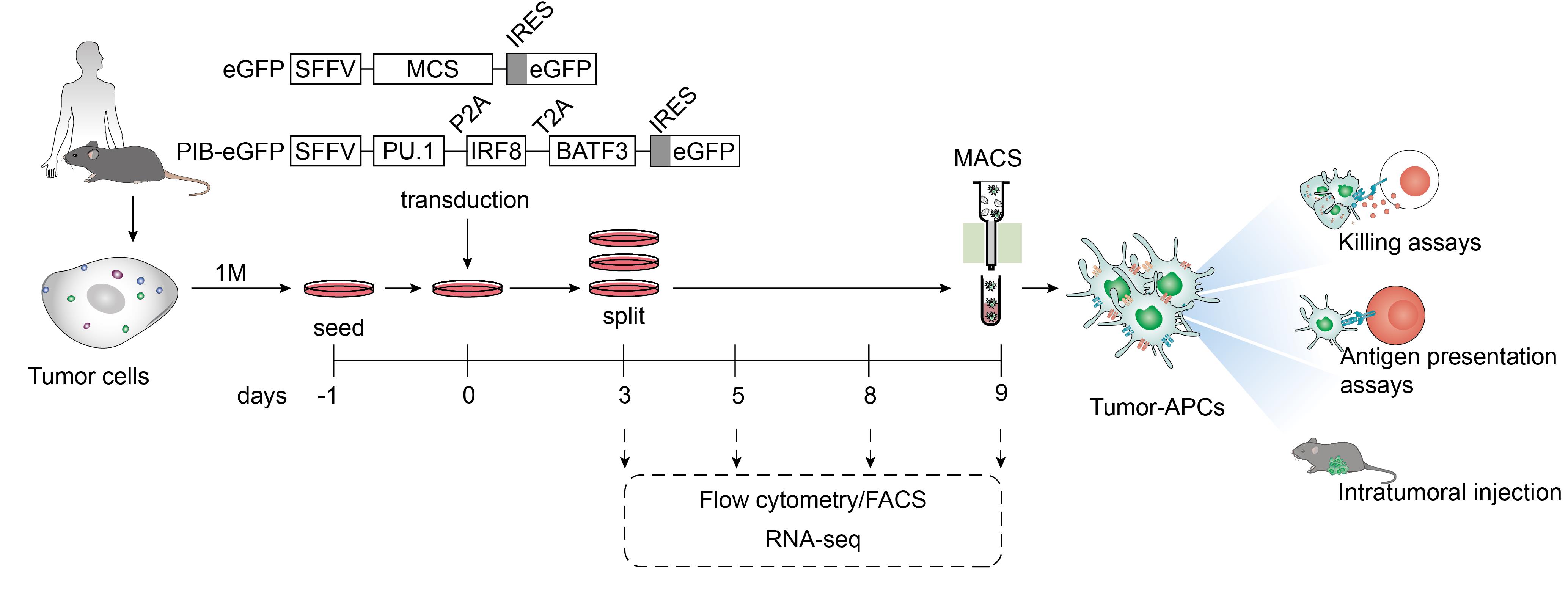
Figure 1. Protocol overview to reprogram mouse and human cancer cells to antigen-presenting dendritic cell-like cells. Tumor cells from murine or human origin are plated one day before transduction with lentiviral particles encoding PU.1, IRF8, and BATF3 in a polycistronic cassette under the control of the spleen focus-forming virus promoter (SFFV). An empty vector with a multiple cloning site (MCS) is used as a control. Both constructs encode eGFP after an internal ribosome entry site (IRES) to trace transduced cells by flow cytometry and fluorescence microscopy. At day 3, confluent plates are split and evaluated for transduction efficiency by flow cytometry. Cells are maintained until day 9 at 60%–80% confluency. Reprogramming efficiency is followed by flow cytometry at days 5, 8, and 9. Tumor-antigen-presenting cells (tumor-APCs) can be purified by fluorescent-activated cell sorting (FACS) for applications that require high purity, including RNA-sequencing (RNA-seq). For in vitro and in vivo functional assessments that require large numbers of reprogrammed cells, tumor-APCs can be enriched by magnetic-activated cell sorting (MACS) through positive selection. To assess enhanced immunogenicity in vitro, tumor-APCs can be co-cultured with activated CD8+ T cell (killing assays). These evaluate whether reprogrammed cancer cells can be targeted by cytotoxic lymphocytes. To evaluate antigen presentation capacity, tumor-APCs are co-cultured with antigen-specific Naïve CD8+ or CD4+ T cells, followed by assessment of T-cell activation and proliferation. In this protocol, we describe antigen presentation to CD4+ T cells to validate tumor-APC function. Moreover, tumor-APCs can be injected intratumorally to evaluate anti-tumor efficacy using tumor growth delay and survival as read-outs.
Materials and reagents
Biological materials
B16F10 cells (B16) (ATCC, catalog number: CRL-6475)
888-mel cells (88MEL) (gifted by Göran B. Jönsson from Lund University)
B6.Cg-Tg (TcraTcrb)425Cbn/J (OT-II mouse) (The Jackson Laboratory, catalog number: 004194) as a source for spleens
C57BL/6j (The Jackson Laboratory, catalog number: 000664) as a source for spleens and bone marrow
Human leucocytes concentrate (Skåne University Hospital, Lund) as a source for PBMCs
Reagents
Plasmid pMD2.G (Addgene, catalog number: 12259)
Plasmid psPAX2 (Addgene, catalog number: 12260)
Plasmid SFFV-mPIB-eGFP containing a tricistronic cassette encoding mouse PU.1, IRF8, and BATF3 (mPIB) (Cell Reprogramming in Hematopoiesis and Immunity Lab, Division of Molecular Medicine and Gene Therapy, Lund University, Sweden)
Plasmid SFFV-hPIB-eGFP containing a tricistronic cassette encoding human PU.1, IRF8, and BATF3 (hPIB) (Cell Reprogramming in Hematopoiesis and Immunity Lab, Division of Molecular Medicine and Gene Therapy, Lund University, Sweden)
Plasmid SFFV-MCS-eGFP empty vector with a multiple cloning site and expressing eGFP (Cell Reprogramming in Hematopoiesis and Immunity Lab, Division of Molecular Medicine and Gene Therapy, Lund University, Sweden)
Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, L-glutamine, and sodium pyruvate (Cytiva, Hyclone, catalog number: SH30243.01)
RPMI 1640 with L-glutamine (Cytiva, Hyclone, catalog number: SH30027.01)
Phosphate buffered saline (PBS) without calcium and magnesium (Cytiva, Hyclone, catalog number: SH30256.01)
Fetal bovine serum (FBS) (Cytiva, Hyclone, catalog number: SV30150.03)
Lenti-XTM qRT-PCR Titration kit (Takara, catalog number: 631235)
Rat serum (GeneTex, catalog number: GTX73226)
Mouse serum (Merck, catalog number: M5905)
GlutaMAXTM Supplement (Thermo Fisher Scientific, Gibco, catalog number: 35050061)
Penicillin-streptomycin solution (Cytiva, Hyclone, catalog number: SV30010)
Sodium pyruvate solution (Thermo Fisher Scientific, Gibco, catalog number: 11360070)
2-Mercaptoethanol (Thermo Fisher Scientific, Gibco, catalog number: 31350010)
Hexadimethrine bromide (Merck, catalog number: H9268-5G)
TrypLE Express (Thermo Fisher Scientific, Gibco, catalog number: 12605010)
Anti-Biotin microbeads (Miltenyi Biotec, catalog number: 130-090-485)
Dead cell removal kit (Miltenyi Biotec, catalog number: 130-090-101)
BD Pharm Lyse lysing buffer (BD Biosciences, catalog number: 555899)
Ammonium chloride solution (Stem Cell Technologies, catalog number: 07800)
Pan-DC Enrichment kit, human (Miltenyi Biotec, catalog number: 130-100-777)
Naïve CD4+ T cell isolation kit, mouse (Miltenyi Biotec, catalog number: 130-104-453)
CellTrace Violet Proliferation kit for flow cytometry (Thermo Fisher Scientific, Invitrogen, catalog number: C34557)
Polyinosinic:polycytidylic acid [poly(I:C)] HMW (InvivoGen, catalog number: tlr-pic-5)
Ovalbumin (OVA) 323–339 (InvivoGen, catalog number: vac-isq)
4′,6′-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific, Invitrogen, catalog number: D1306)
Fixable Viability Dye eFluor eFluor520 (Thermo Fisher Scientific, Invitrogen, catalog number: 65-0867-14)
Anti-mouse CD45 Monoclonal Antibody (clone: 30-F11), Biotin (Thermo Fisher Scientific, eBioscience, catalog number: 13-0451-82)
Anti-mouse CD45 Monoclonal Antibody (clone:30-F11), Allophycocyanin-Cyanine7 (Thermo Fisher Scientific, eBioscience, catalog number: 25-0451-82)
Anti-mouse MHC class II (I-A/I-E) Monoclonal Antibody (clone: M5/114.15.2), Biotin (Thermo Fisher Scientific, eBioscience, catalog number: 13-5321-82)
Anti-mouse MHC class II (I-A/I-E) Monoclonal Antibody (clone: M5/114.15.2), PE-Cyanine7 (Thermo Fisher Scientific, eBioscience, catalog number: 25-5321-82)
Anti-mouse CD274 (PD-L1) Monoclonal Antibody (clone: M1H5), PE, (Thermo Fischer Scientific, eBioscience, catalog number: 12-5982-82)
Anti-mouse CD275 (ICOSL) Monoclonal Antibody (clone: HK5.3), PE (Thermo Fisher Scientific, eBioscience, catalog number: 12-5985-82)
Anti-mouse CD366 (Tim-3) Monoclonal Antibody (clone: B8.2.C12), Allophycocyanin (BioLegend, catalog number: 134008)
Anti-mouse CD3 Monoclonal Antibody (clone: 17A2), FITC (BioLegend, catalog number: 100204)
Anti-mouse/human CD45RA (B220) Monoclonal Antibody (clone: RA3-6B2), FITC (Thermo Fisher Scientific, eBioscience, catalog number: 11-0452-85)
Anti-mouse Ly-6G/Ly-6C (Gr-1) Monoclonal Antibody (clone: RB6-8C5), FITC (BioLegend, catalog number: 108406)
Anti-mouse NK1.1 Monoclonal Antibody (clone: PK136), FITC (Thermo Fisher Scientific, eBioscience, catalog number: 11-5941-82)
Anti-mouse CD8a Monoclonal Antibody (clone: 53-6.7), Allophycocyanin-Cyanine7 (BioLegend, catalog number: 100722)
Anti-mouse CD11c Monoclonal Antibody (clone: N418), Brilliant Violet 650 (BioLegend, catalog number: 117339)
Anti-mouse CD4 Monoclonal Antibody (clone: RM4-5), PE/Cyanine7 (Thermo Fisher Scientific, eBioscience, catalog number: 25-0042-82)
Anti-mouse TCR b chain Monoclonal Antibody (clone: H57-597), Allophycocyanin (BioLegend, catalog number: 109212)
Anti-mouse/human CD44 Monoclonal Antibody (clone: IM7), Allophycocyanin-Cyanine7 (BioLegend, catalog number: 103028)
Anti-human HLA-DR Monoclonal Antibody (clone: L243), PE-Cyanine7 (BioLegend, catalog number: 307616)
Anti-human HLA-DR Monoclonal Antibody (clone: L243), Brilliant Violet 711 (BioLegend, catalog number: 307644)
Anti-human CD45 Monoclonal Antibody (clone: H130), Allophycocyanin-Cyanine7 (BioLegend, catalog number: 304014)
Anti-human CD11c Monoclonal Antibody (clone: B-ly6), Violet 450 (BD Biosciences, catalog number: 560369)
Anti-human CD1c Monoclonal Antibody (clone: L161), Allophycocyanin-Cyanine7 (BioLegend, catalog number: 331520)
Anti-human CD141 (thrombomodulin) Monoclonal Antibody (clone: M80), PE-Cyanine7 (BioLegend, catalog number: 344110)
Anti-human CD3 Monoclonal Antibody (clone: UCHT1), FITC (BioLegend, catalog number: 300452)
Anti-human CD19 Monoclonal Antibody (clone: H1B19), FITC (BioLegend, catalog number: 302206)
Anti-human CD56 (NCAM) Monoclonal Antibody (clone: 5.1H11), FITC (BioLegend, catalog number: 362546)
Anti-human VISTA Monoclonal Antibody (clone: B7H5DS8), Allophycocyanin (Thermo Fisher Scientific, eBioscience, catalog number: 17-1088-42)
Anti-human CD274 (PD-L1) Monoclonal Antibody (clone: M1H1), PE (Thermo Fisher Scientific, eBioscience, catalog number: 12-5983-42)
Anti-human CD366 (TIM3) Monoclonal Antibody (clone: F38-2E2), Allophycocyanin (Thermo Fisher Scientific, eBioscience, catalog number: 17-3109-42)
Lentiviral vectors for expression of PU.1, IRF8, and BATF3 individually in inducible vectors (Addgene, catalog numbers: 139839, 139838, and 139837)
Solutions
OVA 323–339 1 mg/mL, prepared according to manufacturer’s protocol
CellTrace Violet (CTV) solution, prepared according to manufacturer’s protocol
DMEM complete media (see Recipes)
RPMI complete media for 88MEL culture (see Recipes)
RPMI complete media for bone-marrow dendritic cells (BM-DCs) (see Recipes)
MACS buffer (see Recipes)
Polybrene 8 mg/mL (see Recipes)
Poly(I:C) 1 mg/mL, prepared according to manufacturer’s protocol (see Recipes)
Recipes
DMEM complete media
Reagent Final concentration Quantity DMEM high glucose, with glutamine and sodium pyruvate 440 mL Heat-inactivated FBS 10% (v/v) 50 mL GlutaMAX 1% (v/v) 5 mL Penicillin-Streptomycin solution 1% (v/v) 5 mL Total 500 mL RPMI complete media for 88MEL culture
Reagent Final concentration Quantity RPMI 1640 440 mL Heat-inactivated FBS 10% (v/v) 50 mL GlutaMAX 1% (v/v) 5 mL Penicillin-Streptomycin 1% (v/v) 5 mL Total 500 mL RPMI complete media for BM-DCs culture
Reagent Final concentration Quantity RPMI 1640 435 mL Heat-inactivated FBS 10% (v/v) 50 mL GlutaMAX 1% (v/v) 5 mL Penicillin-Streptomycin 1% (v/v) 5 mL Sodium Pyruvate 1% (v/v) 5 mL 2-Mercaptoethanol 0.05 mM 0.5 mL Total 500.5 mL MACS buffer
Reagent Final concentration Quantity PBS without calcium and magnesium 480 mL Heat-inactivated FBS 2% (v/v) 10 mL Penicillin-Streptomycin solution 2% (v/v) 10 mL Total 500 mL Polybrene solution
Reagent Final concentration Quantity Hexadimethrine bromide 8 mg/mL 5 g Milli-Q H2O 625 mL Total 625 mL Poly(I:C) 1 mg/mL solution
Reagent Final concentration Quantity Poly(I:C) 1 mg/mL 10 mg Endotoxin free water (provided by the manufacturer) 10 mL Total 10 mL
Laboratory supplies
Serological pipette, sterile, non-pyrogenic/endotoxin free 2–50 mL (Sarstedt, catalog numbers: 86.1253.001, 86.1256.001, 86.1252.001, 86.1254.001, 86.1685.001)
Pasteur pipette, sterile (Sarstedt, catalog number: 86.1171.001)
1 L vacuum filter system, 0.22 mm PE (Corning, catalog number: 431098)
100 mm tissue culture plates (Corning, catalog number: 430167)
Petri dish, 92 mm × 16 mm (Sarstedt, catalog number: 82.1473)
150 mm tissue culture plates (Corning, catalog number: 430599)
6-well tissue culture plates (Corning, catalog number: 353046)
6-well non-tissue-culture-treated plates (Corning, catalog number: 351146)
96-well tissue culture U-shaped bottom plates (Corning, catalog number: 353077)
96-well non-tissue-culture-treated U-shaped bottom plates (Corning, catalog number: 351177)
15 mL centrifuge tubes (Sarstedt, catalog number: 62.547.205)
50 mL centrifuge tubes (Sarstedt, catalog number: 62.547)
MidiMACSTM LS columns (Miltenyi Biotec, catalog number: 130-042-501)
Hemocytometer chamber for cell counting (Corning, CytoSmart, catalog number: 480202)
Microcentrifuge tubes (Sarstedt, catalog number: 72.690.001)
Cell strainer 45 mm nylon mesh (Corning, catalog number: 11587522)
Syringe 5 mL (Avantor, VWR, catalog number: 613-2042)
Round bottom polystyrene test tube 5 mL (Corning, Falcon, catalog number: 352008)
Tweezers curved ends (Avantor, VWR, catalog number: 232-0106)
Forceps (Avantor, VWR, catalog number: 232-0085)
Scissors (Avantor, VWR, catalog number: 233-0225)
Equipment
Scanlaf Mars class 2 laminar flow hood (LaboGene, model: Mars 1200 mm)
Forma Steri-cycle i160 CO2 incubator (Thermo Scientific, model: i160)
Integra Pipetboy acu 2 (Integra-Biosciences, model: acu 2)
BD FACSymphony A1 or LSRFortessa flow cytometer analyzers (16-color, violet/blue/red/yellow-green) (BD Biosciences, model: A1 Cell Analyzer; LSRFortessa Analyzer)
MACS MultiStand (Miltenyi Biotec, catalog number: 130-042-303)
MidiMACSTM separator (Miltenyi Biotec, catalog number: 130-042-301)
4–16K refrigerated centrifuge (Sigma, catalog number: 10474)
Automatic cell counter (Corning, CytoSmart, catalog number: 6749)
Milli-Q Type 1 Ultrapure Water systems (Merck, model: IQ7000)
Thermo Scientific Finnpipette F2 pipettes (Thermo Fisher Scientific, catalog number: 4642010)
Thermo Scientific Finnpipette F2 Multichannel pipettes (Thermo Fisher Scientific, catalog number: 4662030)
Vortex (IKA, model: MS3 basic)
Water bath (Grant Instruments, model: JB series)
Software and datasets
FACSDIVA (v 9.0) (Access date, 03 May 2023)
FlowJo (v 10.8.1) (Access date, 03 May 2023)
GraphPad Prism (v 9) (Access date, 05 May 2023)
CytoSmart (Cell Count Algorithm V3) (Access date, 01 May 2023)
Mouse Tumor-APC RNA-seq data GSE184527 (Access date, 07 July 2023)
Mouse cDC1 cells RNA-seq data GSE103618 (Access date, 07 December 2018)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ferreira, A. G., Zimmermannova, O., Kurochkin, I., Ascic, E., Åkerström, F. and Pereira, C. F. (2023). Reprogramming Cancer Cells to Antigen-presenting Cells. Bio-protocol 13(22): e4881. DOI: 10.21769/BioProtoc.4881.
- Zimmermannova, O., Ferreira, A. G., Ascic, E., Velasco Santiago, M., Kurochkin, I., Hansen, M., Met, Ã., Caiado, I., Shapiro, I. E., Michaux, J., et al. (2023). Restoring tumor immunogenicity with dendritic cell reprogramming. Sci. Immunol. 8(85): eadd4817.
分类
免疫学 > 免疫细胞功能 > 抗原特异反应
细胞生物学 > 细胞工程 > 部分重编程
癌症生物学 > 肿瘤免疫学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link