- EN - English
- CN - 中文
Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
对早、中、晚草莓进行灰葡萄孢体内接种试验
发布: 2023年10月20日第13卷第20期 DOI: 10.21769/BioProtoc.4859 浏览次数: 2749
评审: Samik BhattacharyaIgnacio Lescano
Abstract
Strawberries are delicious and nutritious fruits that are widely cultivated and consumed around the world, either fresh or in various products such as jam, juice, and ice cream. Botrytis cinerea is a fungal pathogen that causes gray mold disease on many crops, including strawberries. Disease monitoring is an important aspect for growing commercial crops like strawberry because there is an urgent need to develop effective strategies to control this destructive gray mold disease. In this protocol, we provide an important tool to monitor the gray mold fungal infection progression in different developmental stages of strawberry. There are different types of inoculation assays for B. cinerea on strawberry plants, such as in vitro (in/on a culture medium) or in vivo (in a living plant). In vivo inoculation assays can be performed at early, middle, and late stages of strawberry development. Here, we describe three methods for in vivo inoculation assays of B. cinerea on strawberry plants. For early-stage strawberry plants, we modified the traditional fungal disc inoculation method to apply to fungal infection on strawberry leaves. For middle-stage strawberry plants, we developed the flower infection assay by dropping fungal conidia onto flowers. For late-stage strawberry plants, we tracked the survival rate of strawberry fruits after fungal conidia infection. This protocol has been successfully used in both lab and greenhouse conditions. It can be applied to other flowering plants or non-model species with appropriate modifications.
Key features
• Fungal disc inoculation on early-stage strawberry leaves.
• Fungal conidia inoculation on middle-stage strawberry flowers.
• Disease rating for late-stage strawberry fruits.
• This protocol is applicable to the other flowering plants with appropriate modifications.
Graphical overview
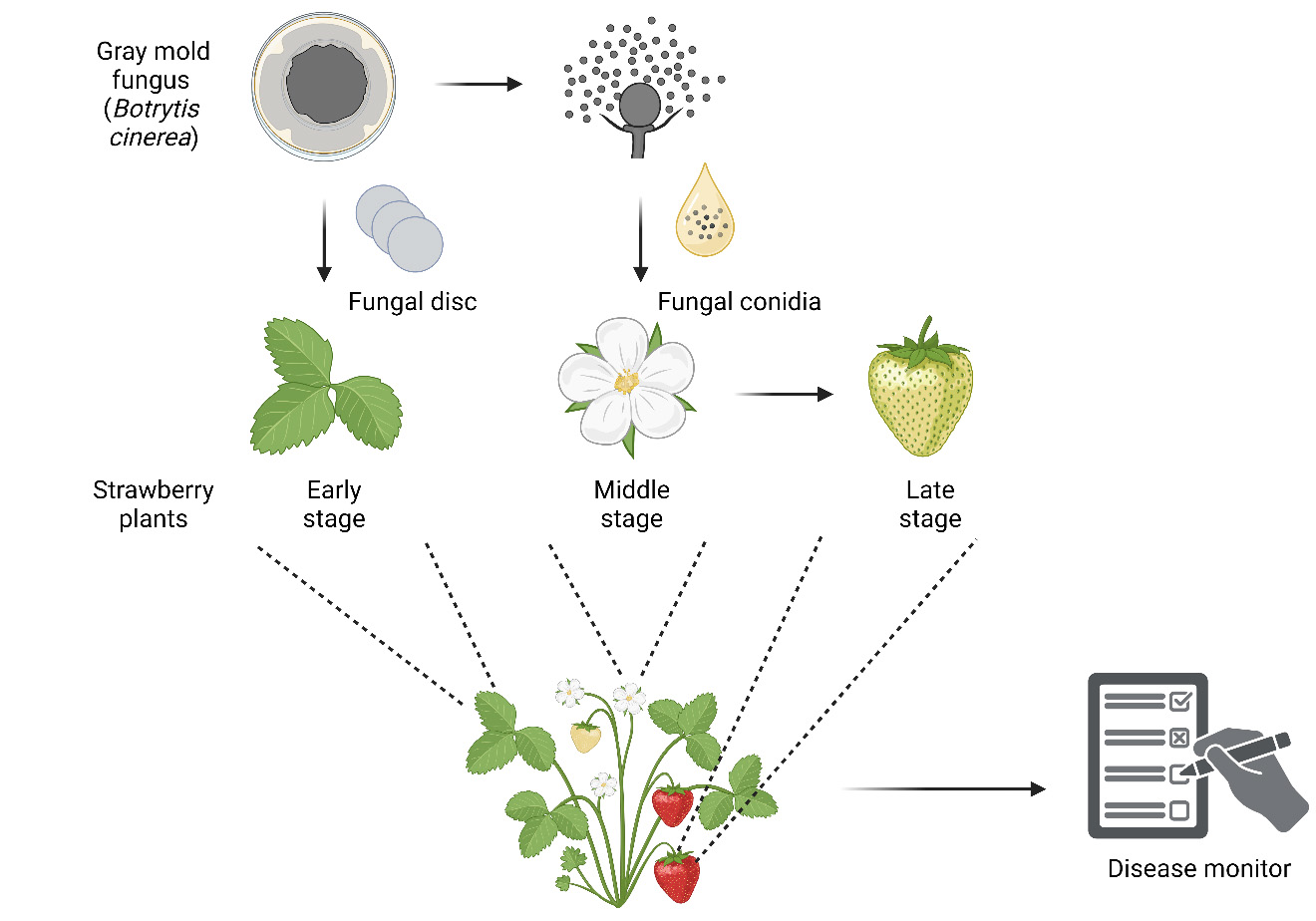
In vivo infection progression assays of gray mold fungus Botrytis cinerea at different developmental stages of strawberry. Created with BioRender.com.
Background
Strawberries are valuable commercial fruit crops that provide critical income and food security for many farmers and consumers around the world. Strawberry plants can be affected by bacterial and fungal infections like gray mold, anthracnose, and powdery mildew, which can damage the plant, reduce fruit quality, and even lead to plant death (Yang et al., 2023a). Botrytis cinerea is the causing agent of the gray mold disease in almost all vegetable and fruit crops, including strawberry plants and post-harvest fruits (Dean et al., 2012; Yang et al., 2023b). This necrotrophic fungal phytopathogen causes an estimated $10–100 billion yearly loss (Fillinger and Elad, 2016). The airborne conidia are produced by B. cinerea in diseased plant tissues, posing a long-lasting threat as part of the secondary infection cycle. An outbreak of the gray mold disease will likely occur under low temperature and high humidity in greenhouse and field conditions. Importantly, B. cinerea is also widely accepted as the second most important fungal pathogen in the research field of molecular plant pathology (Dean et al., 2012), ranked after Magnaporthe oryzae (Chen et al., 2023) but before Fusarium graminearum (Yang et al., 2018). Although plant species such as Arabidopsis and tomato have been used as classic models to study plant response against B. cinerea (Sun et al., 2011), other economically valuable crop species, like strawberries, are not well studied (Petrasch et al., 2019), especially from the flower and fruit perspectives. This protocol provides a detailed method for assessing the response of strawberry plants to the infection by the gray mold fungus B. cinerea at different stages of plant development. It can be adapted for other research purposes (e.g., in vitro detached leaf/fruit inoculation) and other flowering plants with appropriate modifications. The main factors to consider are the inoculation method (fungal disc vs. fungal conidia depending on the plant tissues) and the inoculation dosage (different plant species have varying levels of resistance to the fungus). For example, leaves and fruits of strawberry, tomato, and tobacco can be detached from plants and kept in optimal in vitro conditions (e.g., low temperature and high humidity) for subsequent fungal disc/conidia inoculation. This protocol can also be combined with other methods in related research fields, such as plant–microbe interactions. For instance, some beneficial bacteria can induce plant defense mechanisms that make the plants more resilient to future attacks (Sahib et al., 2019; Zhao et al., 2021; Yang et al., 2022). These defense mechanisms are called induced systemic resistance (ISR) and systemic acquired resistance (SAR) (Chanda et al., 2011; Yang et al., 2023b). This protocol can be used to study ISR/SAR of flowering plants against the gray mold fungus and help develop strategies for plant disease management from the plant’s perspective. However, this protocol has not been tested yet in field conditions and should also be modified according to plant protection principles such as the plant disease triangle, the plant fitness tetrahedron, and the plant disease management hexagon (Yang et al., 2023a), which should take into account multiple factors rather than a single one.
Materials and reagents
B. cinerea strain SS2Ap1 (isolated from diseased strawberry field by IFF)
Petri dishes (90 mm × 14 mm) (VWR, catalog number: 391-0439)
2 mL microcentrifuge tubes (Fisher Scientific, catalog number: 02-681-320)
Sterile water
Lens paper (VWR, catalog number: 52846-000)
Strawberry (day-neutral variety Monterey-UC)
2-gallon pots (greenhouse megastore)
Commercial potting mix (PRO-MIX Company, PRO-MIX BX)
Ziploc sandwich bags (Ziploc, model: 664546)
Spray bottle
Micropore tape (MicroporeTM, catalog number: 1530S-1)
BactoTM agar (BD, catalog number: 214010)
Potato dextrose agar (PDA) (Sigma-Aldrich, catalog number: 70139)
Commercial V8 100% vegetable juice (V8) (Campbell, catalog number: 0067-17KN)
Half-strength PDA medium (see Recipes)
Half-strength V8 medium (see Recipes)
Recipes
Half-strength PDA medium: for culturing B. cinerea
Half-strength of PDA diluted with sterile water.
Half-strength V8 medium: for preparing conidia suspension
Half-strength of supernatant of V8, diluted with sterile water.
Equipment
Autoclave machine (Panasonic Healthcare, model: MLS-3781L)
Sterile pipettes (VWR, catalog number: VHPA23004)
Microcentrifuge (Eppendorf, model: 5415D)
Hemocytometer (Hausser Scientific, catalog number: 497559)
Digital Calipers (Fisher Scientific, catalog number: 1464817)
Light microscope (Nikon, model: E100, required objective: 10×)
Laminar flow hood (SterilGARD 3 Advance)
Freezer (-80 °C) (Panasonic VIP Plus, model: MDF-V76VC-PA)
Vortex mixer (VWR, catalog number: 58815-232)
Laboratory gloves (Fisher Scientific, catalog number: 19-130-1597B)
Forceps (Grainger, catalog number: 4CR15)
Software and datasets
Microsoft Excel Spreadsheet Software (Microsoft 365)
GraphPad Prism (v9.0.2)
All raw example datasets are included in this protocol.
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yang, P., Zhao, Z., Virag, A., Becker, T., Zhao, L., Liu, W. and Xia, Y. (2023). Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries. Bio-protocol 13(20): e4859. DOI: 10.21769/BioProtoc.4859.
分类
植物科学 > 植物免疫 > 病害生物测定
植物科学 > 植物生理学 > 生物胁迫
生物科学 > 生物技术 > 微生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link