- EN - English
- CN - 中文
Maize Seedlings Colonization with Serendipita indica and Its Colonization Efficiency Analysis
玉米幼苗的Serendipita indica定植及其定植效率分析
发布: 2023年10月20日第13卷第20期 DOI: 10.21769/BioProtoc.4855 浏览次数: 2263
评审: Anonymous reviewer(s)

相关实验方案
基于 Emu 的可重复计算流程:在高性能计算集群上实现高保真土壤—植物微生物组解析
Henrique M. Dias [...] Christopher Graham
2026年01月20日 374 阅读
Abstract
Maize is one of the most important crops in the world, and ensuring its successful growth and productivity is crucial for global food security. One way to enhance maize growth and productivity is by improving the colonization of its roots by beneficial microorganisms. In this regard, Serendipita indica, a plant growth–promoting fungus, has gained attention for its ability to enhance plant growth and productivity, especially in cereal crops and medicinal plants. Previous studies have shown that S. indica can colonize various plant species, including maize, but the efficiency of the colonization process in maize seedlings has not been extensively characterized. This protocol outlines a method for efficient colonization of maize seedlings with the beneficial fungus S. indica. The protocol includes the preparation of stock solutions, maintenance and growth of S. indica, surface sterilization and germination of seeds, preparation of S. indica chlamydospores, and colonization of maize plants with S. indica. The advantages of this protocol include the use of surface sterilization techniques that minimize contamination, the production of a large number of viable chlamydospores, and efficient colonization of maize seedlings with S. indica. This protocol may be useful for researchers studying the role of S. indica in promoting plant growth and combating biotic and abiotic stress. Additionally, this protocol may be used in the development of biofertilizers using S. indica as a means of increasing crop yields and reducing dependence on synthetic fertilizers. Overall, this protocol offers a reliable and efficient method for colonizing maize seedlings with S. indica and may have potential applications in the agricultural industry. This study also provides a valuable tool for researchers interested in studying plant–microbe interactions in maize and highlights the potential of S. indica as a biocontrol agent to enhance maize productivity under adverse conditions.
Key features
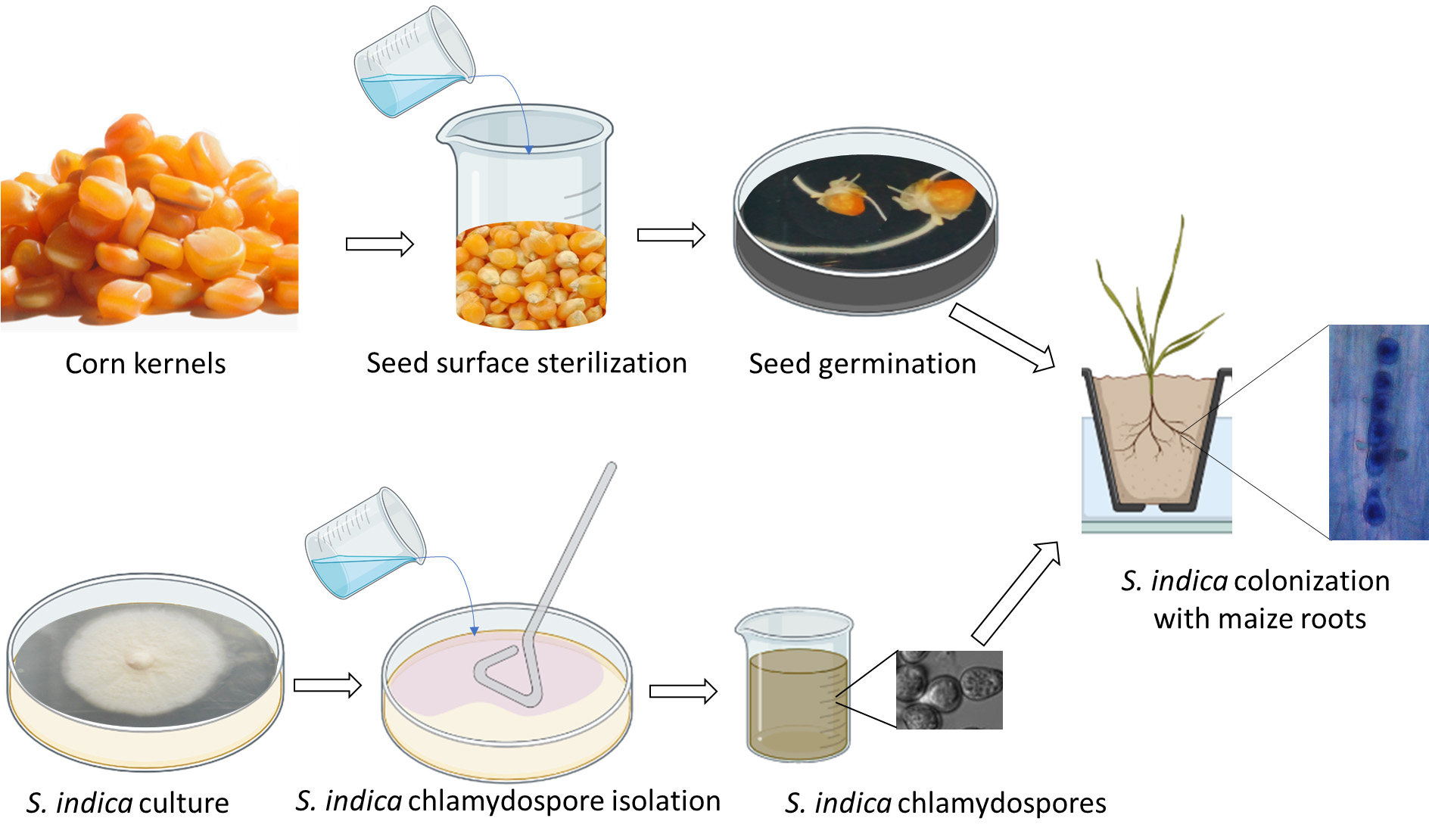
• This protocol builds upon the method developed by Narayan et al. (2022), and its application optimized for the root endophytic symbiotic fungus S. indica.
• This protocol also allows for histochemical analysis to visualize the colonized fungal spores in the root cells of host plant species.
• This protocol helps in mathematical calculation of the percent colonization or efficiency of colonization.
• This protocol utilizes readily available laboratory equipment, including a light microscope, autoclave, and laminar flow hood, ensuring ease of reproducibility in other research laboratories.
Graphical overview
Background
Maize is an important cereal crop and an essential source of food, feed, and biofuel worldwide. However, maize is highly susceptible to biotic and abiotic stresses, such as pests, diseases, and adverse environmental conditions, which can significantly reduce crop yield and quality (Erenstein et al., 2022; Chávez-Arias et al., 2021). Therefore, it is becoming increasingly important to create sustainable and eco-friendly agricultural methods that can improve the productivity and resilience of maize crops. One such strategy is the use of plant growth–promoting fungi (PGPF) to improve plant growth and health (Adedayo and Babalola, 2023).
S. indica (formerly known as Piriformospora indica) is a PGPF that has been reported to enhance the growth and stress tolerance of various plant species, including maize (Singhal et al., 2017; Narayan et al., 2021). The colonization of maize seedlings with S. indica has been shown to increase root growth, nutrient uptake, photosynthetic efficiency, and resistance to various biotic and abiotic stresses (Varma et al., 1999; Narayan et al., 2017, 2021 and 2022; Prasad et al., 2019; Verma et al., 2022). Therefore, the optimization and standardization of the maize seedlings’ colonization protocol with S. indica are crucial to ensure the reproducibility and reliability of the results.
The protocol described in this paper can help explore the mechanisms underlying the beneficial effects of S. indica on maize growth and stress tolerance and identify potential molecular and biochemical markers of plant–fungi symbiosis. Moreover, this protocol can be used to evaluate the performance of different S. indica strains and inoculation methods and to assess the effects of environmental factors on colonization efficiency, and plant growth and development.
Several methodologies have been developed to study the interactions between plant growth–promoting fungi (PGPF) and plants such as wheat (Triticum aestivum), rice (Oryza sativa), barley (Hordeum vulgare), bean (Phaseolus vulgaris), soybean (Glycine max), and chickpea (Cicer arietinum) using S. indica (Rocha et al., 2019; Wahid et al., 2019; El-Maraghy et al., 2020; Mahdi et al., 2022; Verma et al., 2022). One common approach is the use of in vitro culture systems, such as agar dishes or hydroponic cultures, to assess the effects of S. indica on plant growth and stress responses under controlled conditions (Osman et al., 2020). Another approach is the use of field trials to evaluate the performance of S. indica in enhancing crop productivity and quality under natural conditions. Additionally, various molecular and biochemical techniques, such as quantitative polymerase chain reaction (qPCR), metabolomics, and proteomics, were used to analyze the gene and protein expression levels and metabolic pathways involved in the plant–fungi interaction (Gouda et al., 2018).
The protocol described in this paper has several advantages over other published methods (Kumar et al., 2009; Hosseini et al., 2018; Zhang et al., 2018). Firstly, this protocol provides a standardized and reproducible procedure for the colonization of maize seedlings with S. indica. Secondly, this protocol allows for the evaluation of the colonization efficiency and distribution of S. indica in different plant tissues, which provides insights into the spatial and temporal dynamics of the plant–fungi interaction. Thirdly, this protocol can be combined with various physiological, biochemical, and molecular analyses to study the mechanisms underlying the plant–fungi symbiosis.
In addition to the research applications, this protocol has several other possible applications. Firstly, this protocol can be used to develop inoculum production and delivery methods for S. indica, which can be used for commercial purposes, such as biofertilizer or biostimulant production. Secondly, this protocol can be used to screen and identify potential S. indica strains with improved plant growth–promoting and stress tolerance properties, which can be used to develop new and more effective PGPF-based products. Thirdly, this protocol can be used to investigate the interaction between S. indica and other beneficial microbes, such as mycorrhizal fungi or rhizobia, and their combined effects on plant growth and health. Overall, this protocol has the potential to advance research in the field of plant–microbe interactions and to contribute to the development of sustainable and environmentally friendly agricultural practices.
Materials and reagents
Biological materials
Maize seeds (Variety HQPM-5 from Indian Agriculture Research Institute, Pusa, New Delhi, India)
Serendipita indica fungus (strain DSM11827 gifted from Prof. Ajit Verma) (Varma, Kost, Rexer & Franken, 1997, European patent office, Muenchen, Germany. Patent No. 97121440.8-2105, Nov 1998)
Reagents
Potassium hydroxide (KOH) (Sigma-Aldrich, catalog number: 1310-58-3)
Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 7647-01-0)
Phenol (Sigma-Aldrich, catalog number: 108-95-2)
Lactic acid (Sigma-Aldrich, catalog number: 50-21-5)
Glycerol (Sigma-Aldrich, catalog number: 56-81-5)
Trypan blue (Sigma-Aldrich, catalog number: 72-57-1)
Glucose (Sigma-Aldrich, catalog number: 50-99-7)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: 10043-52-4)
Ferrous chloride (FeCl2) (Sigma-Aldrich, catalog number: 7705-08-0)
Calcium nitrate [Ca(NO3)2] (Sigma-Aldrich, catalog number: 13477-34-4)
Magnesium sulfate (MgSO4.7H2O) (Sigma-Aldrich, catalog number: 7487-88-9)
Potassium nitrate (KNO3) (Sigma-Aldrich, catalog number: 7757-79-1)
Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: 7778-77-0)
Boric acid (H3BO3) (Sigma-Aldrich, catalog number: 10043-35-3)
Manganese sulfate monohydrate (MnSO4.H2O) (Sigma-Aldrich, catalog number: 10034-96-5)
Copper sulfate (CuSO4.5H2O) (Sigma-Aldrich, catalog number: 7758-98-7)
Zinc sulfate (ZnSO4.7H2O) (Sigma-Aldrich, catalog number: 7733-02-0)
Ammonium molybdate [(NH4)2MoO4] (Sigma-Aldrich, catalog number: 13106-76-8)
Sodium-hypochlorite (NaClO) (Sigma-Aldrich, catalog number: 7681-52-9)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: 7447-40-7)
Manganese(II) chloride (MnCl2) (Sigma-Aldrich, catalog number: 7773-01-5)
Iron(II) sulfate heptahydrate (FeSO4.7H2O) (Sigma-Aldrich, catalog number: 7782-63-0)
Cobalt(II) chloride (CoCl2) (Sigma-Aldrich, catalog number: 7646-79-9)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 7647-14-5)
Sucrose (Sigma-Aldrich, catalog number: 57-50-1)
Ethylenediaminetetraacetic acid ferric sodium salt (NaFeEDTA) (Sigma-Aldrich, catalog number: 15708-41-5)
Potassium iodide (KI) (Sigma-Aldrich, catalog number: 7681-11-0)
Manganese(II) chloride (MnCl2) (Sigma-Aldrich, catalog number: 7773-01-5)
Sodium molybdate dihydrate (Na2MoO4.2H2O) (Sigma-Aldrich, catalog number:10102-40-6)
Glycine (Sigma-Aldrich, catalog number: 56-40-6)
Ammonium phosphate dibasic [(NH4)2HPO4] (Sigma-Aldrich, catalog number: 7783-28-0)
Iron(III) chloride (FeCl3) (Sigma-Aldrich, catalog number: 7705-08-0)
Ethanol (Sigma-Aldrich, catalog number: 64-17-5)
Peptone (Sigma-Aldrich, catalog number: 73049-73-7)
Yeast extract (Sigma-Aldrich, catalog number: 8013-01-2)
Casamino acid (Sigma-Aldrich, catalog number: 65072-00-6)
Agar (Sigma-Aldrich, catalog number: 9002-18-0)
BD DifcoTM BactoTM Agar (Sigma-Aldrich, catalog number: DF0140-15-4)
Biotin (Sigma-Aldrich, catalog number: 7646-79-9)
Nicotinamide (Sigma-Aldrich, catalog number: 98-92-0)
Pyridoxal phosphate (Sigma-Aldrich, catalog number: 853645-22-4)
Aminobenzoic acid (Sigma-Aldrich, catalog number: 150-13-0)
Riboflavin (Sigma-Aldrich, catalog number: 83-88-5)
Thiamine hydrochloride (Sigma-Aldrich, catalog number: 67-03-8)
Pyridoxine hydrochloride (Sigma-Aldrich, catalog number: 58-56-0)
Nicotinic acid (Sigma-Aldrich, catalog number: 59-67-6)
Trypticase peptone (Sigma-Aldrich, catalog number: 91079-40-2)
Malt extract (Sigma-Aldrich, catalog number: 8002-48-0)
Myo-Inositol (Sigma-Aldrich, catalog number: 87-89-8)
Solutions
Sterile water (1 L)
Double-distilled water (DI water) (1 L)
Half-strength modified Hoagland solution (1 L) (see Recipes)
Ethanol (1 L) 70% (w/v) (see Recipes)
Sodium hypochlorite (NaClO) solution (1 L) 0.75% (w/v) (see Recipes)
0.8% Bacto agar (500 mL) (see Recipes)
Lactophenol (50%) (see Recipes)
Trypan blue (100 mL) (see Recipes)
10% KOH (100 mL) (see Recipes)
Aspergillus modified medium (AMM) or Kaefer (KF) medium (1 L) (see Recipes)
MN-Agar medium (1L) (see Recipes)
Recipes
Half-strength modified Hoagland solution (1 L)
Reagent Final concentration Quantity Macronutrients Ca(NO3)2·4H2O 4 mM 944.5 mg MgSO4·7H2O 2 mM 492.9 mg KNO3 6 mM 606.6 mg KH2PO4 1 mM 136.08 mg Micronutrients H3BO3 45 μM 2.78 mg MnSO4·4H2O 20 μM 3.01 mg CuSO4·5H2O 0.4 μM 99.87 mg ZnSO4·7H2O 0.7 μM 201.32 mg (NH4)2MoO4·2H2O 0.2 μM 41.18 mg H2O 1,000 mL Total 1,000 mL 70% ethanol (1 L)
Reagent Quantity Ethanol (absolute) 700 mL H2O 300 mL Total 1,000 mL 0.75% sodium-hypochlorite (NaClO) (bleach) solution (100 mL)
Reagent Quantity Sodium hypochlorite solution (6%) 12.5 mL ddH2O 87.5 mL Total 100 mL 0.8% Bacto agar (500 mL)
Reagent Quantity Bacto agar 4g ddH2O 500 mL Total 500 mL Lactophenol (50%)
Reagent Quantity Phenol 150 mL ddH2O 150 mL Lactic acid 125 mL Glycerol 125 mL Total 600 mL Trypan blue (100 mL)
Reagent Quantity Trypan blue 0.1 g Lactophenol 100 mL Total 100 mL 10% KOH (100 mL)
Reagent Quantity KOH 10 g ddH2O 100 mL Total 100 mL Aspergillus modified medium/Kaefer medium (1 L)
Reagent Quantity Glucose 20 g Peptone 2 g Yeast Extract 1 g Casamino acid 1 g Vitamin stock solution 1 mL Macro-element stock solution 50 mL Micro-element stock solution 2.5 mL CaCl2 (0.1 M) 1 mL FeCl2 (0.1 M) 1 mL Agar 10 g pH (Adjust with 1 N HCl) 6.5 ddH2O 944.5 mL Total 1,000 mL Macro-elements stock KCl 10.4 g MgSO4·7H2O 10.4 g KH2PO4 30.4 g ddH2O 1,000 mL Total 1,000 mL Micro-elements stock ZnSO4·7H2O 22 g H3BO3 11 g MnCl2·4H2O 5 g FeSO4·7H2O 5 g CoCl2·6H2O 1.6 g CuSO4·5H2O 1.6 g ddH2O 1,000 mL Total 1,000 mL Vitamin stock Biotin 5 mg Nicotinamide 50 mg Pyridoxal Phosphate 10 mg Aminobenzoic Acid 10 mg Riboflavin 25 mg ddH2O 100 mL Total 100 mL 0.1 M FeCl2 FeCl2 1.62 g ddH2O 100 mL Total 100 mL 0.1 M CaCl2 CaCl2 1.11 g ddH2O 100 mL Total 100 mL MN-Agar medium (1 L)
Reagent Quantity NaCl 23.4 mg KNO3 80 mg Ca(NO3)2·4H2O 288 mg Sucrose 10 g NaFeEDTA 8 mg KI 0.8 mg MnCl2·4H2O 6 mg Na2MoO4·2H2O 0.0024 mg Glycine 3 mg KH2PO4 272.2 mg (NH4)2HPO4 40.8 mg CaCl2 81.6 mg MgSO4·7H2O 731 mg FeCl3 583.9 mg Thiamine hydrochloride 67.4 mg Pyridoxine hydrochloride 67.4 mg Nicotinic acid 0.5 mg Trypticase peptone 1 g Glucose 10 g Malt extract 50 g Myo-Inositol 50 mg Bacto agar 10 g KCl 65 mg H3BO3 1.5 mg MnSO4·H2O 6.0 mg ZnSO4·7H2O 2.7 mg CuSO4·5H2O 0.2 mg pH 5.8 Total 1,000 mL Notes:
All the stock solutions are stored at 4 °C and the vitamin stock at -20 °C. The stock of FeSO4·7H2O is prepared separately.
Filter sterilize the vitamin stocks.
Laboratory supplies
Gloves (Genesee Scientific, catalog number: 44-100M)
Lab coat (Fisher Scientific, catalog number: 19-181-570)
Pipettes of different sizes (Fisher Scientific, catalog number: 13-690-029)
Tip boxes (Fisher Scientific, catalog number: 01-670-713)
1 L Glass bottles (Fisher Scientific, catalog number: 13951L)
100 mL Glass bottles (Fisher Scientific, catalog number: 06-414-1A)
Petri dishes 20mm (Fisher Scientific, catalog number: 08-757-099)
Petri dishes 150mm (Fisher Scientific, catalog number: 50-403-868)
Plastic trays (Fisher Scientific, catalog number: 11-394-455)
Blotting papers (Fisher Scientific, catalog number: 09-301-199)
Germination paper (Fisher Scientific, catalog number: NC1466201)
Spreader (Fisher Scientific, catalog number: 14-665-230)
Beaker (Fisher Scientific, catalog number: 07-250-056)
250 mL flask (Fisher Scientific, catalog number: 10-040F)
Surgical blade (Fisher Scientific, catalog number: 22-079-774)
Muslin cloth ( Amazon.in, item model number: HAZC017639)
Nail paint (Amazon.in, item model number: CC4407)
Equipment
Laminar flow hood (Thermo ScientificTM, catalog number: 1323TS)
Light microscope (Leica Microscope, Type 020-518.500, Germany and Nikon Eclipse Ti)
Autoclave (Thymol autoclave, India, product code: TAI-902)
Incubator shaker (Multitron Incubator Shaker, HT-Infors, Switzerland)
Glass house (School of Life Sciences, Jawaharlal Nehru University, New Delhi India)
Software and datasets
Microsoft Office Excel 10
GraphPad Prism 8
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Narayan, O. P., Yadav, B., Verma, N., Dua, M. and Johri, A. K. (2023). Maize Seedlings Colonization with Serendipita indica and Its Colonization Efficiency Analysis. Bio-protocol 13(20): e4855. DOI: 10.21769/BioProtoc.4855.
分类
植物科学 > 植物免疫 > 宿主-细菌相互作用
植物科学 > 植物生理学 > 生物胁迫
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link