- EN - English
- CN - 中文
Princeton RAtlas: A Common Coordinate Framework for Fully cleared, Whole Rattus norvegicus Brains
Princeton RAtlas:完全清除的完整褐家鼠大脑的通用坐标框架
发布: 2023年10月20日第13卷第20期 DOI: 10.21769/BioProtoc.4854 浏览次数: 1796
评审: Oneil Girish BhalalaXiaochen SunEmma Puighermanal
Abstract
Whole-brain clearing and imaging methods are becoming more common in mice but have yet to become standard in rats, at least partially due to inadequate clearing from most available protocols. Here, we build on recent mouse-tissue clearing and light-sheet imaging methods and develop and adapt them to rats. We first used cleared rat brains to create an open-source, 3D rat atlas at 25 μ resolution. We then registered and imported other existing labeled volumes and made all of the code and data available for the community (https://github.com/emilyjanedennis/PRA) to further enable modern, whole-brain neuroscience in the rat.
Key features
• This protocol adapts iDISCO (Renier et al., 2014) and uDISCO (Pan et al., 2016) tissue-clearing techniques to consistently clear rat brains.
• This protocol also decreases the number of working hours per day to fit in an 8 workday.
Graphical overview
Background
Recent developments in tissue clearing and imaging allow us to gather whole-brain connectivity in intact brains, together with classic neuronal tracing techniques.Though tissue fixation and preparation predated neuroscience, its use was refined by Golgi and further modified and popularized by Ramón y Cajal (1911). To this day, after experiments, neuroscientists often remove the brain and slice it into sections that are then mounted to microscope slides for imaging. This allows for both post-hoc verification of experimental targeting and, if paired with staining or immunohistochemistry, learning about the tissue. However, this process is hard to automate and time-consuming: each step of fixation, removal, slicing, and imaging for each slice can take hours. Additional immunohistochemistry or staining can take weeks. To decrease the amount of labor required to process each brain, researchers will frequently only keep a subset of the brain slices near the relevant regions of study. Often this is more than sufficient, but this approach can easily miss potentially relevant information in the discarded sections, and sectioning itself can introduce information and aberrations to data that are difficult to identify post-hoc. Address this issue, several groups have developed methods to clear the lipids and pigments from whole brains, allowing for imaging through the entire tissue without the need for sectioning and often dramatically decreasing imaging time.
Building on advances from the early 1900s, tissue clearing has experienced a resurgence in neuroscience over the last 20 years. Methods like CUBIC (Susaki et al., 2015), CLARITY (Chung and Deisseroth, 2013), FluoClearBABB (Schwarz et al., 2015), and the DISCO methods (Renier et al., 2014; Pan et al., 2016) have become popular with researchers studying mice (see Molbay et al., 2021 for a review) yet those of us studying rats have not yet fully embraced this new methodology. One major impediment for broad acceptance of these methods in larger-brained animals is that existing clearing techniques do not consistently produce fully cleared, intact brains, prohibiting the study of subcortical structures in whole brains and decreasing their utility over slice histology. However, several advances have been made: Woo and colleagues nearly doubled the length of the mouse-centric CUBIC method to 20 days to improve clearing rats (Woo et al., 2018); Branch and colleagues successfully cleared entire rat brains by splitting samples into two hemispheres before clearing, together with increasing the timing of stages of the mouse iDISCO protocol (Branch et al., 2019); and a third method, FluoClearBABB, cleared the majority of tissue from an intact rat brain in 8 days while controlling the pH during dehydration, which, similar to uDISCO, preserved fluorescence, though the clearing at the center of the tissue is unclear from their manuscript (Stefaniuk et al., 2016).
Similar advances in imaging techniques allow for faster image acquisition. Typically, slices are imaged on confocal or 2-photon microscopes, which use point scanning methods that scan across and through tissues to excite and observe fluorescence. In contrast, light-sheet microscopy images planes instead of points, decreasing the time needed to go through the same volume of tissue. Like other methods key to modern developments, light-sheet microscopy also was first proposed in 1902 (Siedentopf and Zsigmondy, 1902), but recent developments in CMOS sensors that can acquire large images quickly, as well as advances in light sculpting (Keller et al., 2008), moving illumination and detection (Ahrens et al., 2013), and others [see review Hillman et al. (2019)] were key in making whole-brain imaging available to neuroscientists.
Inspired by these advances, we set out to produce two reliable, worker-friendly protocols for efficient clearing of intact rat brains, based on the uDISCO and iDISCO protocols. Coupled with light-sheet imaging, we can now image intact rat brains, including subcortical structures. We use these clear brains to form a light-sheet-based rat brain template for the rat neuroscience community.
Whole-brain clearing of Rattus norvegicus brains
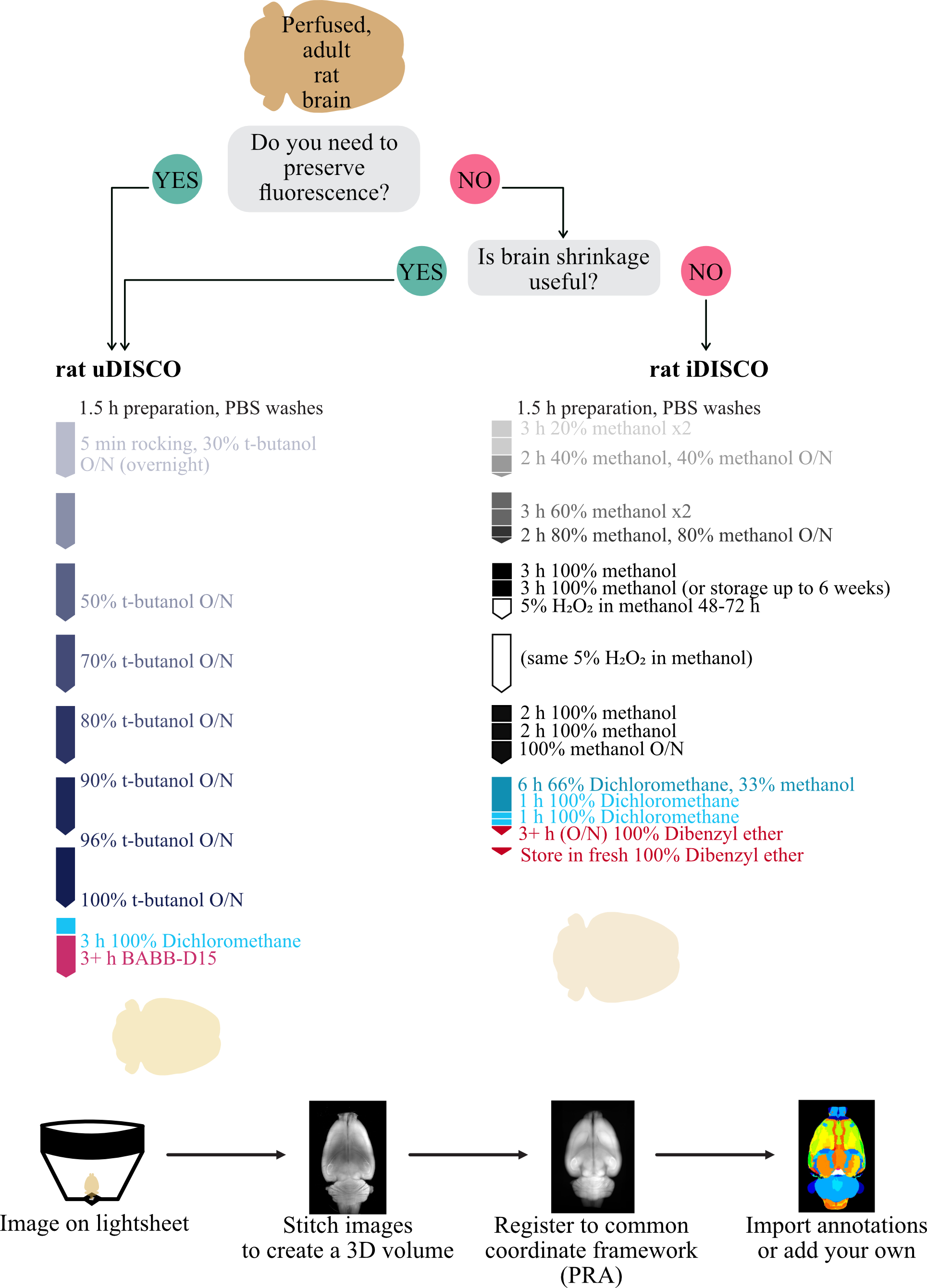
We first set out to improve existing clearing techniques to work consistently on whole adult Rattus norvegicus brains of any size or sex. Inspired by many, we primarily focused on the DISCO techniques and the timing of stages, as this was the most effective in our preliminary explorations. We successfully made a worker-friendly protocol (Figure1A, maximum 8 h days, compared with 10–16 h days in other protocols) that also resulted in consistently clear brains, visible in the field-standard qualitative evaluations (Figure1B and 1C) and a newer quantitative evaluation method, Fourier ringcorrelation–based quality estimation (FRC-QE) (Figure 1E) (Preusser et al., 2021). When applied appropriately, FRC-QE allows for comparable measurements across experiments and provides quantitative data in addition to the field-standard qualitative data.
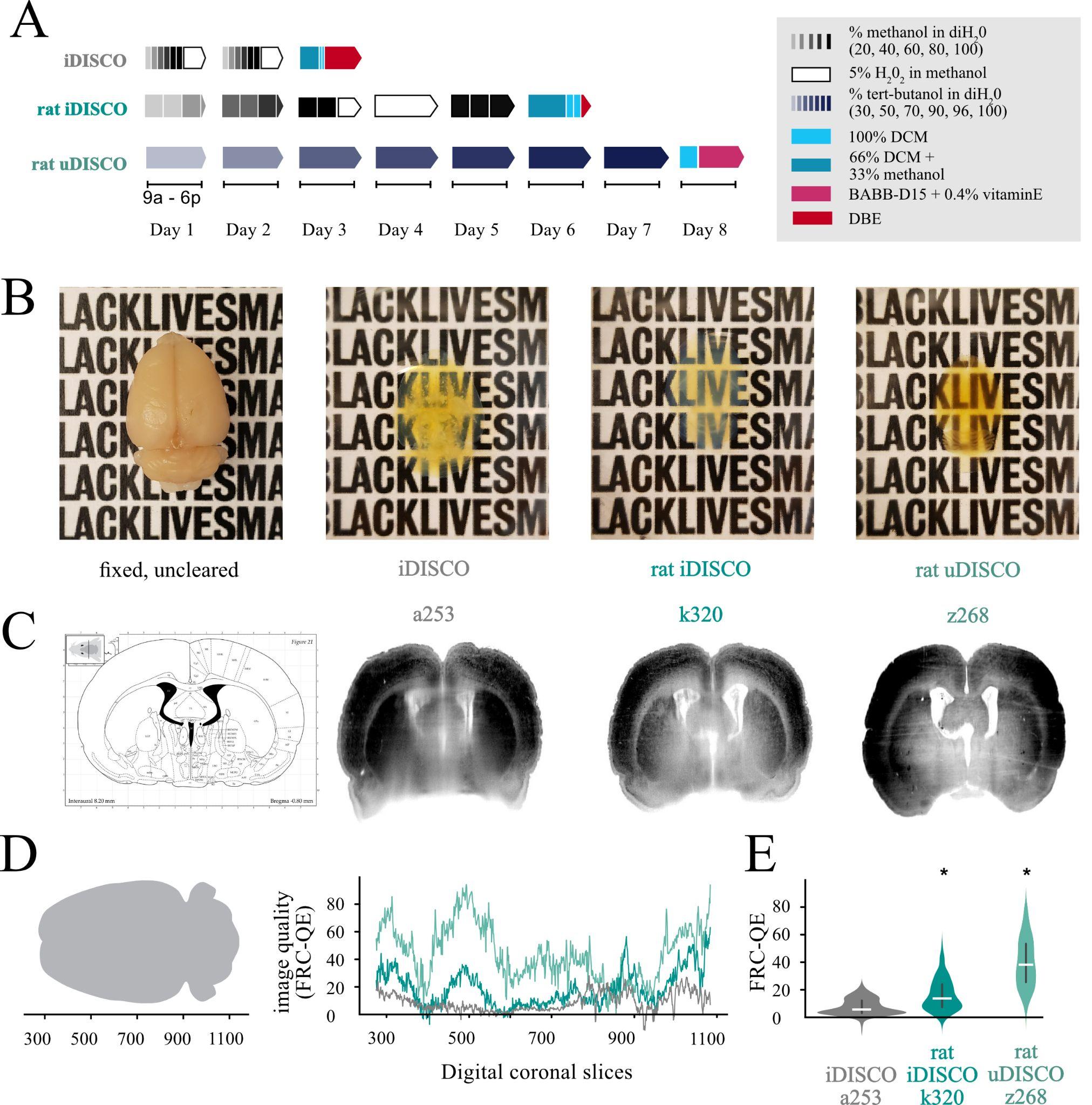
Figure 1. Modifications to iDISCO and uDISCO protocols allow for fully cleared ratbrains. A. Schematics of iDISCO protocol and the modified rat iDISCO and uDISCOprotocols (abbreviations DCM, BABB, and DBE all refer to solutions inRecipes). B. Example images of brains before clearing and after clearing with each protocol from the schematic. The uncleared brain is approximately 2.5 cm long and 1.7 cm wide. All images were taken on the same piece of paper for easy comparison. C. Computational slices through each clearedbrain in B. Labels (a253, k320, z268) reflect the animal’s name. D. Drawing showing the orientation of the brain (left) and the FRC-QE scores (right) for each brain in B along the coronal axes in slice units. E. Quantification of the values in (D), with a Bonferroni-corrected t-test. Horizontal white bars are median values, and the violin limits are the 10% (bottom) and 90% (top) of the data. Asterisks indicate significance p < 0.05 compared with iDISCO a253.
Princeton RAtlas
Once we could reliably obtain fully cleared brains, we set out to make a common coordinate framework for rats using whole-brain, light-sheet imaging. We imaged 18 brains and chose the best eight for an averaged template brain. We chose these eight based on a manual inspection of quality and based on external genitalia: we chose four male and four female brains without obvious damage or imaging aberrations, like streaking. The four female brains were all of sufficient quality that we did not need to procure additional brains, and we chose four of the male brains to match our number of females. We used an iterated, paired averaging scheme to create the seed brain (Figure 2A) and then aligned each brain to this seed, averaged the result to create the new seed, and repeated this process two additional times. The final averaged result of all brains aligned to this final seed brain became the Princeton RAtlas (PRA, Figure 2B). This coordinate space is agnostic to the sex of the animal. We also provide mPRA, a common coordinate space using only animals with visible penis and testes, and fPRA, a common coordinate space using only animals without visible penis or testes.
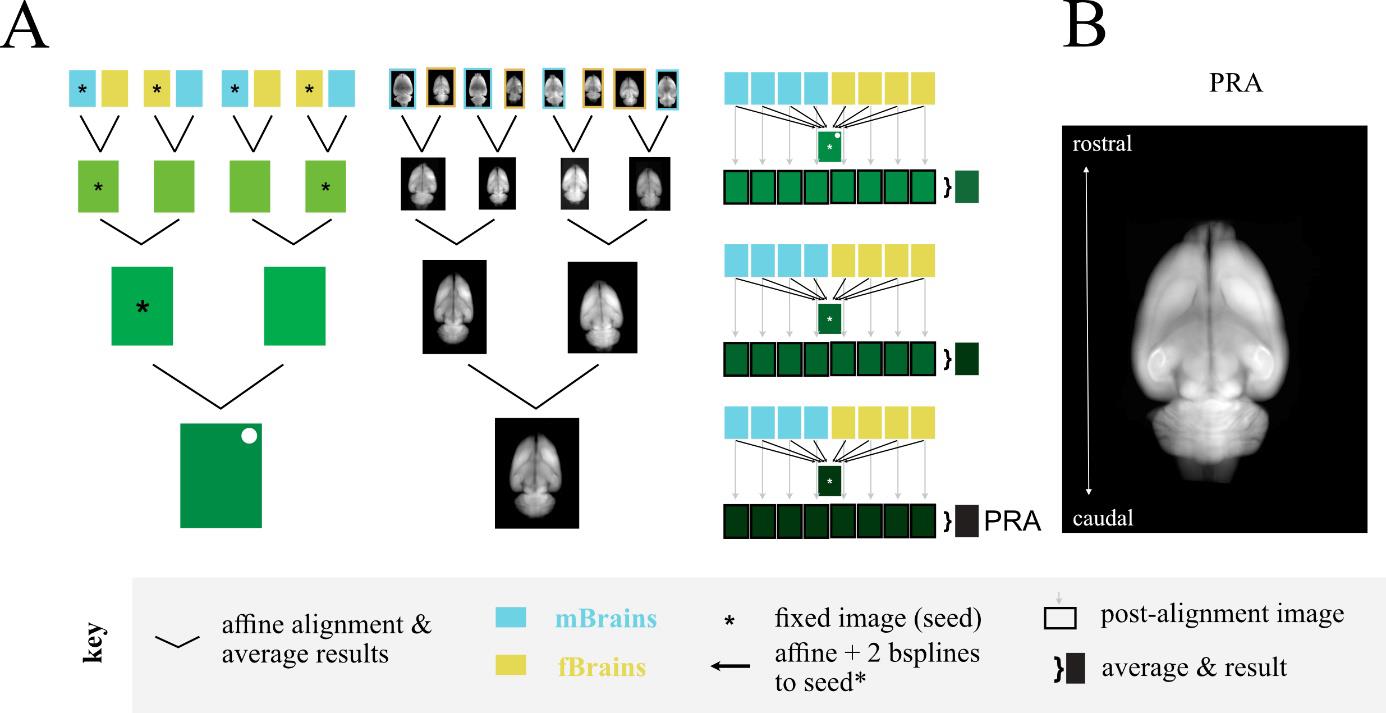
Figure 2. Creation of the Princeton RAtlas (PRA). A. Schematics showing the paired alignments to create the seed brain (left, middle) and the multilevel refinement resulting in the PRA (right). B.Summed axial projection of the PRA. mBrains and fBrains are male and femalebrains respectively, defined by external genitalia.
Materials and reagents
Biological materials
Long-Evans rats [Hilltop Lab Animals Inc., Long Evans Hla(LE)CVF, Scottdale, PA] of any sex. Animals used in this study were 3–12 months and fed ad libitum from 290 to 597 g, though older and larger animals have since been successfully cleared but are not included in this focused manuscript.
Reagents
Ketamine (MedVet International, catalog number: RXKETAMINE, CAS: 6740-88-1)
Xylazine (MedVet International, catalog number: RXXYLAZINE, CAS: 7361-61-7)
Isoflurane (shopmedvet.com, catalog number: RXISO-100)
Phosphate buffered saline (PBS) (Thermo Fisher Scientific, catalog number: 14190136)
Heparin (Patterson Veterinary, catalog number: 07-805-8262, CAS 9005-49-6)
4% paraformaldehyde in PBS (Fisher Scientific, catalog number: AAJ19943K2)
Methanol (Carolina Biological Supply, catalog number: 874195, CAS: 67-56-1)
30% Hydrogen peroxide (Sigma-Aldrich, catalog number: H1009, CAS: 7722-84-1)
Dichloromethane (DCM) (Millipore-Sigma, catalog number: 270997-2L, CAS: 75-09-2)
Benzyl ether (DBE) (Sigma-Aldrich, catalog number: 108014-1KG, CAS: 103-50-4)
Vitamin E (A17039 DL-alpha-Tocopherol, 97%+) (Alfa Aesar, catalog number: 10191-41-0, CAS: 10191-41-0)
Benzyl alcohol (Sigma-Aldrich, catalog number: W213705, CAS: 100-51-6)
Benzyl benzoate (Sigma-Aldrich, catalog number: B9550, CAS: 120-51-4)
Diphenyl ether (DPE 99%) (A15791, 101-84-8, Alfa Aesar, Lancashire, UK, CAS: 101-84-8)
Tert-butanol (Sigma-Aldrich, catalog number: 471712, CAS: 75-65-0)
(Optional) Sodium azide (Sigma-Aldrich, catalog number: S2002, CAS: 26628-22-8)
Solutions
Methanol/diH2O (see Recipes)
70% ethanol (see Recipes)
Peroxide and methanol solution (see Recipes)
Dichloromethane (DCM) 66%/33% Methanol (see Recipes)
Tert-butanol in diH2O (see Recipes)
Benzyl alcohol:benzyl benzoate (BABB) (see Recipes)
BABB-D15 (see Recipes)
1× PBS (see Recipes)
Recipes
Recipes can be scaled up to clear multiple brains in parallel. The volumes below are for a single brain.
Methanol/diH2O series
Make these fresh the day of or the day before, and allow them to reach room temperature, which can take 1.5 h.
Reagent Final concentration Volume Methanol 20%, 40%, 60%, 80% 4 mL, 8 mL, 24 mL, 16 mL diH2O n/a 16 mL, 12 mL, 8 mL, 4 mL Total n/a 20 mL 70% ethanol (higher concentrations are ok, used to wipe down and remove DBE)
This can be made ahead and stored for up to a year if in a sealed container.
Reagent Final concentration Volume Ethanol (absolute) 70% 700 mL H2O n/a 300 mL Total n/a 1,000 mL Peroxide and methanol solution
This can be made ahead and stored for up to a year if in a sealed container.
Reagent Final concentration Volume 30% Hydrogen Peroxide (H2O2) 5% 4 mL Methanol 80% 20 mL Total n/a 24 mL 66% Dichloromethane (DCM) and 33% methanol solution
CAUTION: Use double or chemical nitrile gloves, work under hood or snorkel, expel slowly. This should be made within 24 h and not stored long term because of its volatility.
Reagent Final concentration Volume Dichloromethane 66% 16 mL Methanol 33% 8 mL Total n/a 24 mL Tert-butanol solutions
Make within 24 h and ideally 1.5 h ahead of use. Allow to reach 37 °C, which can take 1.5 h. In some labs, room temperature is too cold for tert-butanol, and it can solidify at higher concentrations. If so, bring up to 37 °C to make dilutions and for use either in an incubator or water bath. If your lab is too cold for liquid tert-butanol, also bring the brains up to 37 °C as described in the procedure.
Reagent Final concentration Volume Tert-butanol 30%, 50%, 70%, 80%, 90%, 96% 7.2, 12, 16.8, 19.2, 21.6, 23.04 mL diH2O n/a 16.8, 12, 7.2, 4.8, 2.4, 0.96 mL Total n/a 24 mL BABB (Benzyl alcohol benzyl benzoate)
This can be made ahead and stored for up to a year if in a sealed container. Other groups have successfully replaced BABB with ethyl cinnamate (Henning, 2019), which is less toxic.
Reagent Final concentration Volume Benzyl alcohol 33.33% 6 mL Benzyl benzoate 66.66% 12 mL Total 100% 18 mL BABB-D15
This can be made ahead and stored for up to a year if in a sealed container.
Reagent Final concentration Volume BABB (see above) 93.38% 15 mL Diphenyl ether (DPE) 6.22% 1 mL Vitamin E 0.4% 0.064 mL Total n/a 16.064 mL 1× PBS
This can be made ahead and stored for up to a year if in a sealed container. Recommend adding 0.1% sodium azide and/or storing at 4 °C if possible, but not required.
Reagent Final concentration Volume 10× Phosphate Buffered Saline 10% 50 mL diH2O 90% 450 mL Total 500 mL
Laboratory supplies
Rongeurs (Fine Science Tools, catalog number: 16022-15)
20 mL scintillation vials (Millipore Sigma, catalog number: Z190535)
3D-printed holder (we used a Form3 SLA 3D-printer and Black Resin from Formlabs, catalog number: RS-F2-GPBK-04) https://github.com/emilyjanedennis/PRA/blob/main/rat_brainholder.STL
Objective MI Plan 1.1×, NA 0.1 (Miltenyi Biotec, catalog number: 150-000-493)
Filter (Semrock, catalog number: FF01-525/39-25)
5 mL Eppendorf tubes (Genesee Scientific, catalog number: 86-899SC)
Super glue. We used Loctite Super Glue (Loctite, catalog number: 212111) but any similar product should be acceptable.
Equipment
Rocker (Genesee Scientific, model: 27-529)
Ultramicroscope II (LaVision Biotec. Bielefeld, Germany)
LifeCanvas SmartSPIM (LifeCanvas SmartSPIM, Cambridge, MA)
Fume hood/snorkel
Perfusion equipment. This should not be sensitive to brand or type. If you see a lot of capillaries in your cleared brains, this usually means that the perfusion is incomplete. We used MasterFlex L/S (NA5183975, VWR, Radnor, PA) and tubing size 16 (3.10 mm ID). Please also see Gage et al. (2012)
Access to a workstation computer and/or a computational server or cluster. There are no specific requirements, but the more powerful the computer, the faster each step will take. We recommend at least an Intel i7 Processor and 64 GB of available RAM or equivalent. More RAM, processing cores, and faster processor speeds will produce noticeable improvements
Waxholm Space Atlas (WHS) https://www.nitrc.org/projects/whs-sd-atlas from Papp et al. (2014)
Software and datasets
ImSpector Microscope controller software Version 5.1.347 (free)
Abberior Instruments Development Team, Imspector Image Acquisition & Analysis Software v16.3, http://www.imspector.de
Elastix 4.8 or 5.0 (free)
Custom software (free) http://www.github.com/emilyjanedennis/PRA Scripts are written in Python (3.0+). The server-side implementation is also written in bash (2.0+)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Dennis, E. J., Bibawi, P., Dhanerawala, Z. M., Lynch, L. A., Wang, S. S. H. and Brody, C. D. (2023). Princeton RAtlas: A Common Coordinate Framework for Fully cleared, Whole Rattus norvegicus Brains. Bio-protocol 13(20): e4854. DOI: 10.21769/BioProtoc.4854.
分类
神经科学 > 神经解剖学和神经环路 > 荧光成像
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link