- EN - English
- CN - 中文
Three-dimensional Co-culture Model for Live Imaging of Pancreatic Islets, Immune Cells, and Neurons in Agarose Gel
用于琼脂糖凝胶中胰岛、免疫细胞和神经元实时成像的三维共培养模型
(§ Technical contact) 发布: 2023年10月20日第13卷第20期 DOI: 10.21769/BioProtoc.4852 浏览次数: 2533
评审: Mohan BabuAndrea GramaticaShun Yu Jasemine YangAnonymous reviewer(s)
Abstract
During the onset of autoimmune diabetes, nerve–immune cell interactions seem to play an important role; however, there are currently no models to follow and interfere with these interactions over time in vivo or in vitro. Two-dimensional in vitro models provide insufficient information and microfluidics or organs on a chip are usually challenging to work with. We present here what we believe to be the first simple model that provides the opportunity to co-culture pancreatic islets with sympathetic nerves and immune cells. This model is based on our stamping device that can be 3D printed (STL file provided). Due to the imprint in the agarose gel, sympathetic neurons, pancreatic islets, and macrophages can be seeded in specific locations at a level that allows for confocal live-cell imaging. In this protocol, we provide the instructions to construct and perform live cell imaging experiments in our co-culture model, including: 1) design for the stamping device to make the imprint in the gel, 2) isolation of sympathetic neurons, pancreatic islets, and macrophages, 3) co-culture conditions, 4) how this can be used for live cell imaging, and 5) possibilities for wider use of the model. In summary, we developed an easy-to-use co-culture model that allows manipulation and imaging of interactions between sympathetic nerves, pancreatic islets, and macrophages. This new co-culture model is useful to study nerve– immune cell– islet interactions and will help to identify the functional relevance of neuro-immune interactions in the pancreas.
Key features
• A novel device that allows for 3D co-culture of sympathetic neurons, pancreatic islets, and immune cells
• The device allows the capture of live interactions between mouse sympatheticneurons, pancreatic islets, and immune cells in a controlled environment after six days of co-culturing.
• This protocol uses cultured sympathetic neurons isolated from the superior cervical ganglia using a previously established method (Jackson and Tourtellotte, 2014) in a 3D co-culture.
• This method requires 3D printing of our own designed gel-stamping device (STL print file provided on SciLifeLab FigShare DOI: 10.17044/scilifelab.24073062).
Graphical overview
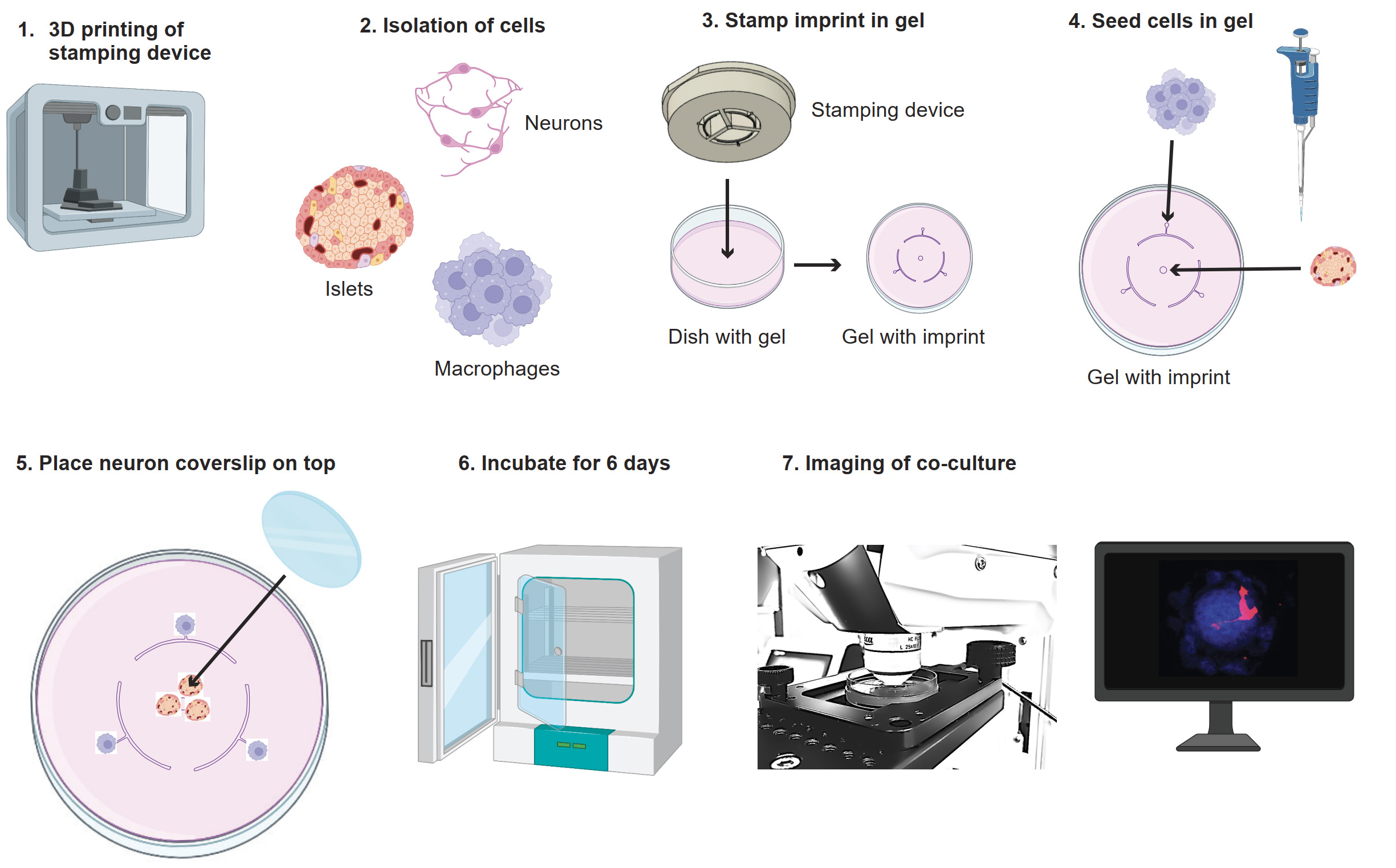
Graphical overview of co-culture model. 1) Print the stamp with a 3D printer. 2) Isolate neurons, islets, and macrophages. 3) Use the stamp to make the imprint in the agarose gel. 4) Seed the macrophages and islets in the agarose gel on their seeding points. 5) Place the coverslip with neurons on top. 6) Incubate the culture for six days. 7) Image the co-culture. Images adapted from BioRender.
Background
It is becoming increasingly clear that the sympathetic nervous system and its interactions with immune cells play an important role in maintaining homeostasis(Martinez-Sanchez et al., 2022). In line with this, type 1 diabetes (T1D) onset in mice halts after depletion of macrophages or sympathetic nerves, via surgical denervation or chemical ablation of nerves (Christoffersson et al., 2020). This shows that sympathetic nerves and macrophages play important roles in T1D onset. Moreover, less severe autoimmune diabetes onset was observed in non-obese diabetic mice (NOD) when the pancreatic nerve was stimulated (Guyot et al., 2019). This suggests that nerve signal modulation might be beneficial for T1D patients in the future and needs to be investigated further. However, currently there are no models to follow and interfere in pancreatic nerve–immune cell interactions over time in vivo.
Injection of neuromodulating agents such as neuropeptides, neurotransmitters, and receptor antagonists in mice has systemic effects, limiting isolation of microenvironmental changes. Separate culture and stimulation of cells in 2D cultures will, on the other hand, provide insufficient information. Microfluidics or organs on a chip are solutions used to measure hormone secretion and more complex cellular behavior in co-culture conditions, but the setup for these microfluidic models is usually challenging. Therefore, we developed a simple 3D co-culture model that allows imaging of interactions between sympathetic nerves, pancreatic islets, and macrophages.
In this protocol, we provide the instructions to construct and perform live cell imaging experiments in our co-culture model. We describe: 1) the design for the stamping device to make the imprint in the gel, 2) the isolation of sympathetic neurons, pancreatic islets, and macrophages, 3) co-culture conditions, and 4) how this can be used for live cell imaging. Finally, we discuss possibilities for a wider use of the model and suggestions for processing of the gel containing islets and cells after imaging for further data collection.
Using this new model, it is possible to study and interfere in neuro-immune interactions in the context of the pancreatic islet in a controlled environment. This environment can be manipulated by addition of neurotransmitters, peptides, or drugs. Instead of macrophages, it would be possible to seed other immune cells, such as T cells, dendritic cells, or monocytes. Due to the three seeding points for cells, it is also possible to seed different immune cells at the same time for example inflammatory and anti-inflammatory macrophages or differently activated Tcells. The versatility of this model makes it useful for studying pancreatic islet physiology and immune attack. In the future, it could also be adapted for the use of pancreatic islet organoids in combination with human immune cells. Altogether, this new co-culture model is useful to study nerve–immune cell–isletinteractions and will help to identify the functional relevance of neuro-immune interactions in the pancreas.
Materials and reagents
Biological materials
Two-day-old pups from a DsRed mouse litter [JAX strain 005441, Tg(CAG-DsRed*MST)1Nagy/J]
Mice expressing GFP [JAX strain 011106, Tg(CAG-GFP*)1Hadj/J]
Wildtype mice (Jackson Laboratory, C57BL/6 mice)
Reagents
Collagenase A, Type 4 (Roche, catalog number: 11088793001), use 10 mg/mL in media
12 mm poly-D-lysine(PLL)/laminin coated German Glass coverslip (Corning BioCoat, catalog number: 354087)
Nerve growth factor (NGF) (Peprotech, catalog number: 450-01)
Trypsin 0.25% EDTA, Mg2+ Ca2+-free (Corning Cellgro®, catalog number: 25-053-Cl)
Fetal bovine serum (FBS) (Sigma, catalog number: F7524-500ML)
Neurobasal media (Gibco, catalog number: 21103049)
B27 supplement (Gibco, catalog number: 17504044)
Penicillin-streptomycin (Pen/strep) 10,000 U/mL (Gibco, catalog number: 15140122)
UltraPure agarose (Invitrogen, catalog number: 1650010)
Low-gelling temperature agarose (Sigma, catalog number: A9045-5G)
RPMI 1640 medium (Thermo Fisher, catalog number: 21875-091)
D-(+)-Glucose (VWR, AnalaR NORMAPUR, catalog number: 101174Y)
Celltracker violet (Invitrogen, catalog number: C10094)
Macrophage colony stimulating factor (M-CSF) (Peprotech, catalog number: 3512-02)
L-glutamine (Life Technologies, catalog number: 25030-024)
Milli-Q (tapped from Purelab Ultra, model: USC214628)
Solutions
Neuron medium (see Recipes)
Islet medium (see Recipes)
Co-culture medium (see Recipes)
Agarose gel (see Recipes)
Low-gelling agarose gel (see Recipes)
Recipes
Neuron medium
Reagent Final concentration Quantity Neurobasal media - 492.8 mL B27 supplement 2% 200 μL Pen/strep 0.5% 2.5 mL L-glutamine 0.5 mM 5 mL Islet medium
Reagent Final concentration Quantity RPMI1640 435 mL FBS 10% 50 mL Pen/Strep 5% 5 mL L-Glutamine 0.5 mM 5 mL D-(+)-Glucose 11.1 mM [1 g in 5 mL] 5 mL Co-culture medium
Reagent Final concentration Quantity Islet medium (from recipe 2) - 50 mL M-CSF 50 ng/mL 50 μL NGF 10 ng/mL 50 μL *M-CSF and NGF are not stable; keep frozen as aliquots (add shortly before use)
Agarose gel
Reagent Final concentration Quantity UltraPure agarose 1.2% 0.48 g Autoclaved Milli-Q
RPMI1640 medium
-
-
10 mL
30 mL
Low-gelling agarose gel
Reagent Final concentration Quantity Low-gelling temperature agarose 1.2% 0.48 g Autoclaved Milli-Q
RPMI 1640 medium
-
-
10 mL
30 mL
Laboratory supplies
6 cm tissue culture dishes (VWR, Avantor, catalog number: 10861-588)
10 cm tissue culture dishes (Thermo Scientific, catalog number: 150464)
96-well U-bottom tissue culture plate (VWR, catalog number: 734-2328)
25 cm cell scraper (VWR, catalog number: 734-2602)
50 mL tubes (Falcon, catalog number: 352070)
50 mL Erlenmeyer (VWR, catalog number: 214-1130)
Parafilm M (VWR, catalog number: 291-1214)
Water bath (Grant, catalog number: TC120)
Equipment
Surgical stereo microscope (Leica, model: MZ10F)
Brightfield microscope (VWR, model: 020025)
Fluorescent microscope (Bio-Rad, ZOE Fluorescent Imager, model: 1450031)
Confocal microscope (Leica, model: TCS SP8, objective model: HC Fluotar L 25×/0.95 W)
FormTM Steri-CylceTM CO2 incubator (Thermo Fisher, model: 371)
Laminar flow hood (Kojair, model: KBS-125)
Microwave (Whirlpool, model VIP 20)
Heating device for imaging (LCI custom heating plate for 6 cm dishes)
3D printer (Asiga, model: MAX X 27)
Cell counting chamber (Neubauer, model: HIRS8100204)
Benchtop centrifuge (Beckman Coulter, model: Allegra X30R)
Software and datasets
ImageJ/Fiji for analyzing obtained imaging data, version number: 1.8.0 (free software)
Microscopy image analysis software, IMARIS, version number: 9.9 (requires a license)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Muntjewerff, E. M., Josyula, V. S. and Christoffersson, G. (2023). Three-dimensional Co-culture Model for Live Imaging of Pancreatic Islets, Immune Cells, and Neurons in Agarose Gel. Bio-protocol 13(20): e4852. DOI: 10.21769/BioProtoc.4852.
分类
细胞生物学 > 细胞分离和培养 > 3D细胞培养
神经科学 > 细胞机理 > 细胞分离和培养
免疫学 > 免疫细胞成像 > 共聚焦显微镜技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link