- EN - English
- CN - 中文
Cell Cycle Analysis of Candida albicans by Flow Cytometry
通过流式细胞仪分析白色念珠菌的细胞周期
(*contributed equally to this work) 发布: 2023年10月20日第13卷第20期 DOI: 10.21769/BioProtoc.4848 浏览次数: 2331
评审: Alba BlesaAnonymous reviewer(s)

相关实验方案
一种基于流式细胞术的利用基因编码荧光报告测定酵母活细胞内pH值的方法
Catherine G. Triandafillou and D. Allan Drummond
2020年06月20日 4822 阅读
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1435 阅读
Abstract
The cell cycle is a vital process of cell division that is required to sustain life. Since faithful cell division is critical for the proper growth and development of an organism, the study of the cell cycle becomes a fundamental research objective. Saccharomyces cerevisiae has been an excellent unicellular system for unraveling the secrets of cell division, and the process of synchronization in budding yeast has been standardized. Cell synchronization is a crucial step of cell cycle analysis, where cells in a culture at different stages of the cell cycle are arrested to the same phase and, upon release, they progress synchronously. The cellular synchronization of S. cerevisiae is easily achieved by a pheromone or other chemicals like hydroxyurea treatment; however, such methodologies seem to be ineffective in synchronizing cells of multimorphic fungi such as Candida albicans. C. albicans is a human pathogen that can grow in yeast, pseudohyphal, and hyphal forms; these forms differ in morphology as well as cell cycle progression. More importantly, upon subjecting to DNA replication inhibitors for synchronization, C. albicans develops hyphal structures and grows asynchronously. Therefore, here we describe a simple and easy method to synchronize C. albicans cells in the G1 phase and the subsequent analysis of cell cycle progression by using flow cytometry.
Keywords: Cell synchronization (细胞同步)Background
Cell cycle is a multievent cellular process that plays an essential role in maintaining genome stability by coordinating processes like DNA replication, DNA damage repair, and chromosome segregation during cell division. The whole cycle is completed in four phases, where DNA replication is confined to the S phase, G1 is the gap between the M phase and S phase, G2 is the gap between the S phase and M phase, and the division of the nucleus followed by the cytoplasm is restricted to the M phase (Figure 1). G1 and G2 phases are known for cell growth, metabolic activity, and for preparing for the next phase of the cell cycle (Hartwell et al., 1974). When the cells are proliferating, they can be at any phase of the cell cycle; therefore, to properly explore the mechanism of cell cycle progression, synchronization of a heterogeneous population of cells becomes critical. In human cells, serum starvation and double thymidine block are commonly used approaches for synchronizing a cell population (Ma and Poon, 2017). Saccharomyces cerevisiae cells can be synchronized in the G1 phase of the cell cycle with the mating pheromone, α-factor. Similarly, hydroxyurea and nocodazole synchronize budding yeast cells in the early S phase and G2/M phase, respectively (Rosebrock, 2017).
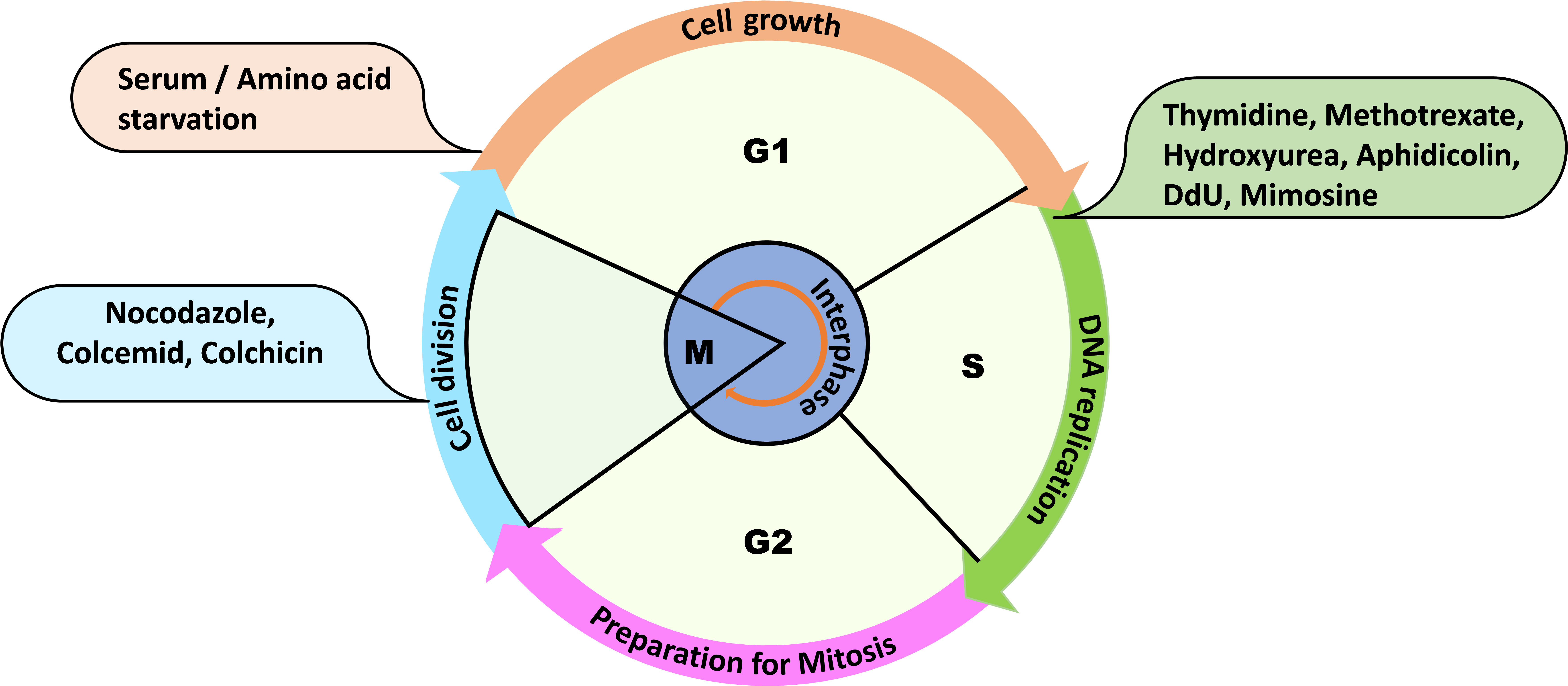
Figure 1. Overview of the cell cycle phases and some routinely used reagents for cell synchronization at the mentioned phases of the cell cycle. For example: thymidine, methotrexate, hydroxyurea, aphidicolin, dedoxy uridine, and mimosine are used to synchronize the cells at G1 or early S phase.
Upon releasing these synchronized cells into a fresh medium, cells undergo similar cell cycle progression that can be monitored after collecting cell populations at different time points. DNA content measurement of a cell population by using DNA-binding fluorescent dyes and a flow cytometer is one of the reliable techniques for getting insights into the cell cycle dynamics of an organism (Haase and Reed, 2002). The existing methodologies of cell synchronization do not work effectively in the case of fungi like Candida albicans. C. albicans is a gut pathogen that survives in at least three morphological forms: yeast, pseudohyphae, and hyphae. Interestingly, these cell types differ even in the rate and order of cell cycle events (Berman, 2006). In addition, most of these reagents used to synchronize cells also induce filamentation in C. albicans (Manohar et al., 2018; Kumari et al., 2023; Patel et al., 2023). Therefore, cell synchronization is one of the major challenges for exploring the cell cycle in C. albicans. Additionally, the use of external chemical reagents for cell synchronization may affect the native cellular physiology by inducing stress in the cells, which will never be a true estimation of the cell cycle and end up in erroneous cell cycle analyses. Therefore, here we describe and demonstrate a chemical method/approach where a time-point-based incubation results in > 90% cell synchronization at the G1 phase so that the cell cycle analysis can be executed without hampering the native physiology of the cells (Figure 2 and Supplementary Figure 1).
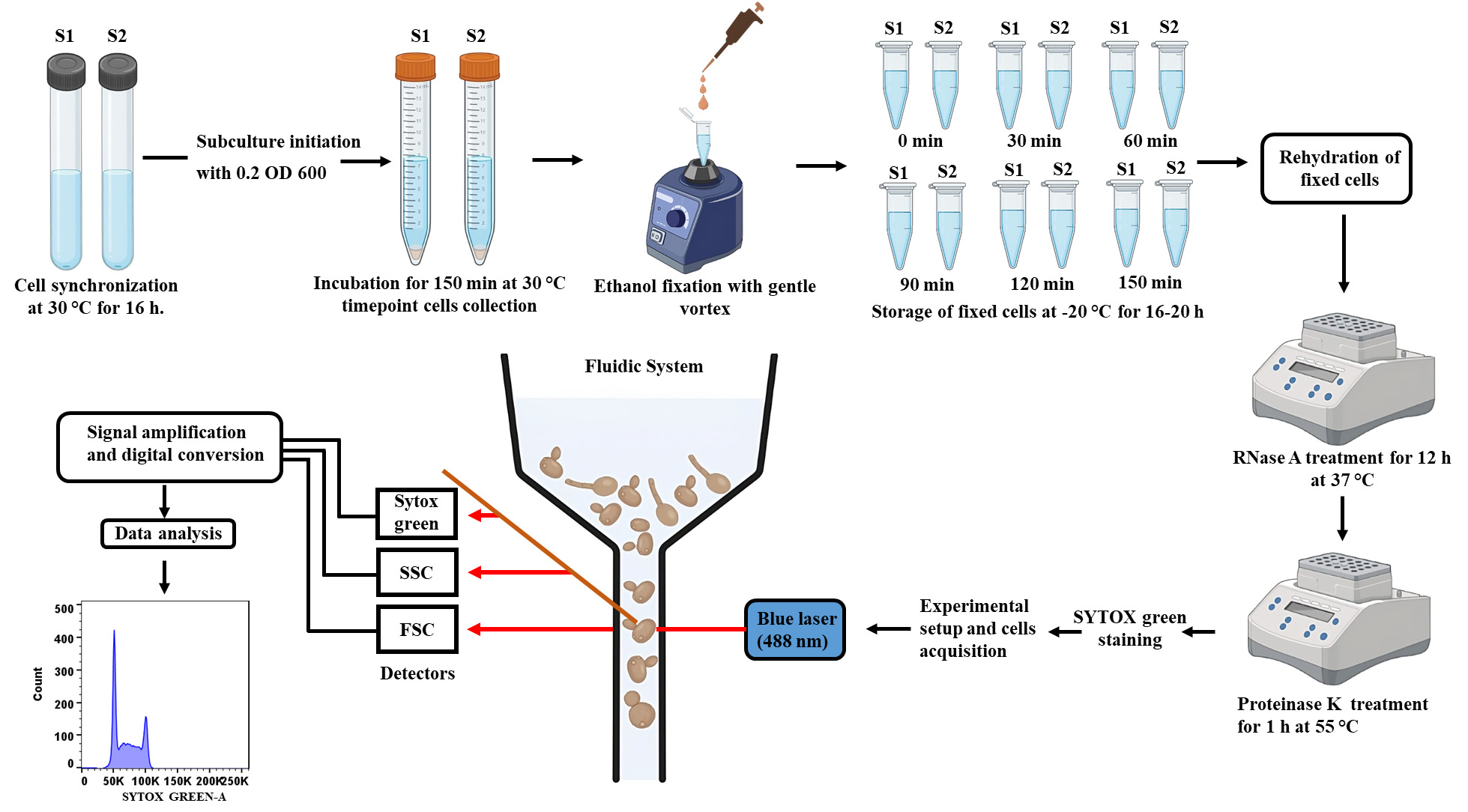
Figure 2. Schematic stepwise demonstration of the cell cycle analysis of C. albicans. Synchronized cells will be allowed to grow for 150 min and will be harvested at every 30 min to monitor cell cycle progression. Cells will be fixed with ethanol, stained with SYTOX green, and analyzed by flow cytometry to complete the cell cycle analysis.
One of the key advantages of using flow cytometry for cell cycle analysis in yeast is its ability to analyze a large number of cells at a single-cell level rapidly. This technique employs a laser-based system to measure the fluorescence emitted upon a fluorochrome (in this case, a nucleic acid staining dye) binding to cellular DNA while cells pass through a flow cell. Different nucleic acid staining dyes including 4′,6-diamidino-2-phenylindole (DAPI), propidium iodide (PI), and 7-amino actinomycin D (7-AAD) have been used by researchers to access the cell cycle based on the cell types to be analyzed, although PI is the preferred one. In S. cerevisiae, PI staining has been proven effective, and the same was initially adopted by us for cell cycle analysis of C. albicans (Manohar et al., 2018). However, a major concern associated with PI is the relatively high coefficient of variation (CV) and low signal amplification rate of PI-stained cells, which limit the accuracy and reproducibility of the cell cycle (Haase and Reed, 2002). To overcome this problem, we now use SYTOX green as it exhibits low CV, high signal amplification with low signal-to-noise ratio, and low photobleaching rate, thus being an ideal fluorochrome for accurate and reproducible DNA content measurement (Thakur et al., 2015; Patel et al., 2023). In summary, this protocol outlines detailed steps involved in the cell cycle analysis of a wild-type strain of C. albicans, SC5314, using SYTOX green with a simple cell synchronization strategy providing the precise measurement of DNA content in each phase of the cell cycle by flow cytometry (Figure 3). By employing this protocol, researchers can gain valuable insights into the cell cycle dynamics, which will be helpful in understanding the diverse physiological processes of C. albicans. The same protocol can be adopted for other fungal species as well.
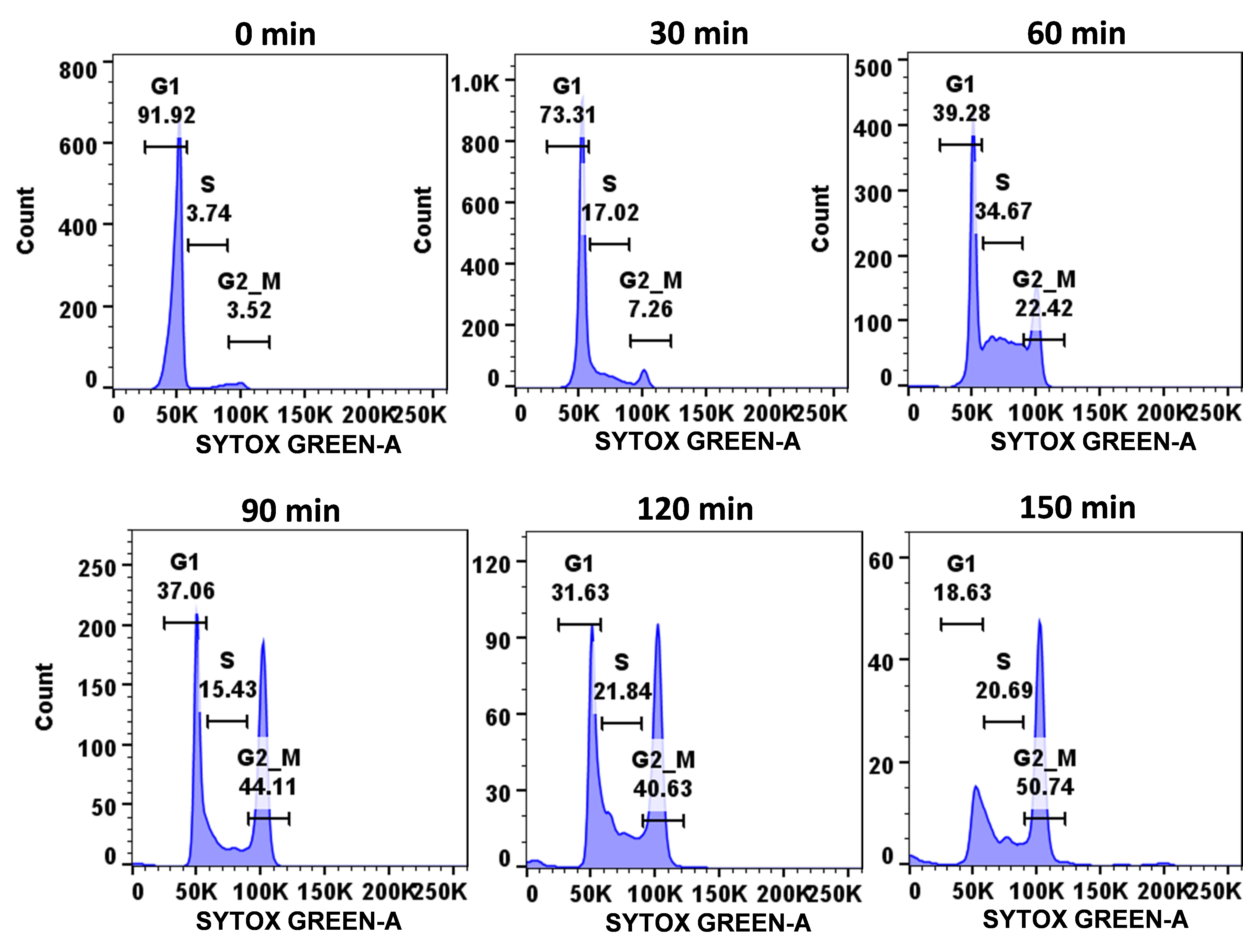
Figure 3. Demonstration of cell cycle progression of wild-type C. albicans cells. Using the described protocol, a synchronized population of C. albicans cells (SC5314 strain) was allowed to progress through the cell cycle and, at mentioned time points (0–1 h), cells were harvested, fixed, stained with SYTOX green, and analyzed by flow cytometry.
Materials and reagents
C. albicans SC5314 or any other strain of interest
Autoclavable round bottom glass culture tubes (Borosil, catalog number: 9900006)
Yeast extract peptone dextrose broth (YPD) (Himedia, catalog number: M1363)
Ethanol (Fisher Chemical, catalog number: 2051537)
Sterile 1.5 mL microcentrifuge tube (Axygen, catalog number: MCT150LC)
FACS tube (Tarson, catalog number: 850010)
Cuvettes (Eppendorf, catalog number: 0030106300)
Proteinase K (Puregene, catalog number: PG-6070)
RNase A (SRL, catalog number: 98915)
Sodium citrate (Mp Biomedical, catalog number: 194817)
SYTOX green (Thermo Fisher Scientific, catalog number: S7020)
Dimethyl sulfoxide (DMSO) (Mp Biomedical, catalog number: 196055)
Acetic acid glacial 99%–100% (Merck, catalog number: CE1C710249)
Tris (Mp Biomedical, catalog number: 194855)
CaCl2 (Sigma-Aldrich, catalog number: C4901-500G)
Glycerol (Himedia, catalog number: MB060-1L)
NaCl (Mp Biomedical, catalog number: 152575)
Solutions
Sodium citrate buffer (see Recipes)
Proteinase K solution (see Recipes)
RNase A solution (see Recipes)
Recipes
Sodium citrate buffer
Reagent Final concentration Quantity Sodium citrate 50 mM 14.7 g H2O n/a Make up the volume Total n/a 1,000 mL Adjust the pH to 7.4 with glacial acetic acid.
Proteinase K solution
Reagent Final concentration Quantity Proteinase K 20 mg/mL 100 mg Tris-HCl (1 M, pH 8.0) 10 mM 50 μL CaCl2 (100 mM) 1 mM 50 μL Glycerol 50% 3.125 mL H2O n/a 1.775 mL Total n/a 5 mL Store the solution in 1 mL aliquots in 1.5 mL microcentrifuge tubes at -20 °C.
RNase A solution
Reagent Final concentration Quantity RNase A 10 mg/mL 200 mg Tris-HCl (1 M, pH 7.5) 10 mM 200 μL NaCl (5 M) 15 mM 60 μL H2O n/a 19.54 mL Total n/a 20 mL Boil the solution at 95 °C for 15 min and allow it to cool slowly at room temperature. Store the solution in 1 mL aliquots in 1.5 mL microcentrifuge tubes at -20 °C.
Equipment
Laminar airflow (Thermo Scientific Biological Safety Cabinets, catalog number: 41346502)
Sterile pipette sets (Gilson, catalog number: F123606-1mL, F123605-200µL, and F123604-20µL)
Spectrophotometer (Eppendorf Bio Photometer plus, catalog number: 6132)
Shaker incubator 30 °C (Scigenic Biotech, catalog number: LE-4676-AA)
Incubator 30 °C (Scigenic Biotech, catalog number: C-1NC-100-1)
Centrifuge (Eppendorf, catalog number: 5810R with SL086 rotor)
Flow cytometer (BD LSRFortessa Cell Analyzer, catalog number: 647177H6)
-20 °C freezer (Vestfrost, catalog number: BFS 345)
Heat block (Eppendorf Thermo Mixer C, catalog number: 5382000023)
Software
BD FACSDiva Software
FlowJo v8.2.0
GraphPad prism v8.0
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Patel, S. K., Sahu, S. R. and Acharya, N. (2023). Cell Cycle Analysis of Candida albicans by Flow Cytometry. Bio-protocol 13(20): e4848. DOI: 10.21769/BioProtoc.4848.
分类
微生物学 > 微生物细胞生物学
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link