- EN - English
- CN - 中文
Production, Extraction, and Solubilization of Exopolysaccharides Using Submerged Cultures of Agaricomycetes
利用伞菌深层培养物生产、提取和溶解胞外多糖
发布: 2023年10月05日第13卷第19期 DOI: 10.21769/BioProtoc.4841 浏览次数: 2186
评审: Lucy XieOsarenkhoe Omorefosa OsemwegieAnonymous reviewer(s)
Abstract
Macrofungi, also known as mushrooms, can produce various bioactive compounds, including exopolysaccharides (EPS) with distinct biological properties and subsequent industrial applications in the preparation of cosmetics, pharmaceuticals, and food products. EPS are extracellular polymers with diverse chemical compositions and physical properties secreted by macrofungi in the form of capsules or biofilms into the cellular medium. Submerged cultivation is an industrially implemented biotechnological technique used to produce a wide variety of fungal metabolites, which are of economic and social importance due to their food, pharmaceutical, and agronomic applications. It is a favorable technique for cultivating fungi because it requires little space, minimal labor, and low production costs. Moreover, it allows for control over environmental variables and nutrient supply, essential for the growth of the fungus. Although this technique has been widely applied to yeasts, there is limited knowledge regarding optimal growth conditions for filamentous fungi. Filamentous fungi exhibit different behavior compared to yeast, primarily due to differences in cell morphology, reproductive forms, and the type of aggregates generated during submerged fermentation. Furthermore, various growing conditions can affect the production yield of metabolites, necessitating the development of new knowledge to scale up metabolite production from filamentous fungi. This protocol implements the following culture conditions: an inoculum of three agar discs with mycelium, agitation at 150 rpm, a temperature of 28 °C, an incubation time of 72 h, and a carbon source concentration of 40 g/L. These EPS are precipitated using polar solvents such as water, ethanol, and isopropanol and solubilized using water or alkaline solutions. This protocol details the production procedure of EPS using submerged culture; the conditions and culture medium used are described. A detailed description of the extraction is performed, from neutralization to lyophilization. The concentrations and conditions necessary for solubilization are also described.
Key features
• Production and extraction of EPS from submerged cultures of mycelial forms of macrofungi.
• Modification of the method described by Fariña et al. (2001), extending its application to submerged cultures of mycelial forms of the macrofungi.
• Determination of EPS production parameters in submerged cultures of mycelial forms of macrofungi.
• EPS solubilization using NaOH (0.1 N).
Graphical overview
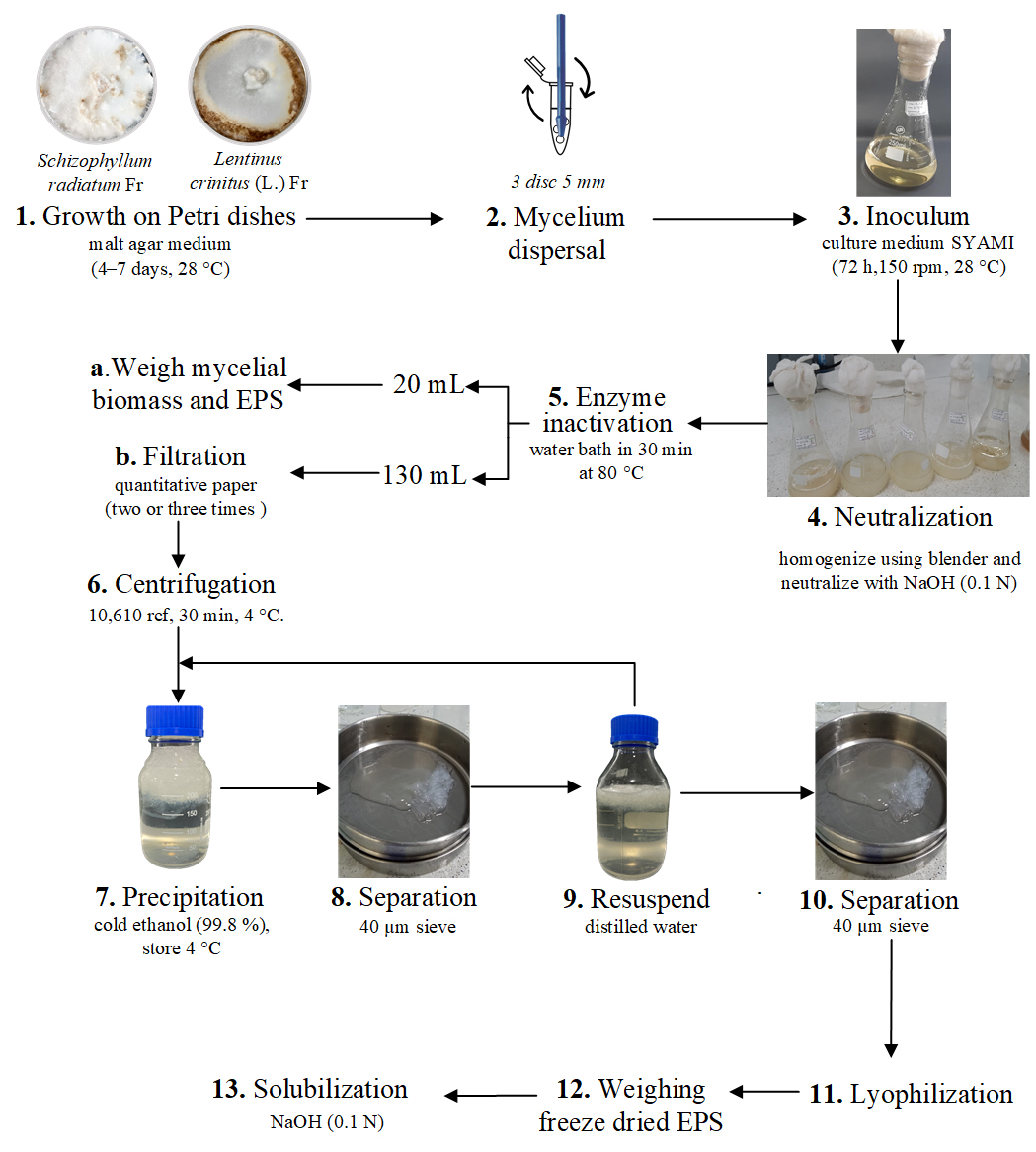
Background
Macrofungi are fungi that produce reproductive bodies that are visible to the naked eye. Some macrofungi known for their production of polysaccharides are the Shiitake (Lentinula eodes) or the mushroom turkey tail (Trametes versicolor). Macrofungal polysaccharides are a group of compounds of great interest in bioprospecting studies of both medicinal and edible fungi, owing to their broad spectrum of bioactivities, including cytotoxic, antiproliferative, antitumor, immunomodulatory, anti-inflammatory, antioxidant, and stimulatory effects, on the colon microflora (Arora and Tandon, 2015; X. Meng, 2016; Nowakowski et al., 2021). These bioactivities are related to the chemo-preventive action of macrofungal polysaccharides against different types of cancers such as colorectal cancer and other chronic diseases. Macrofungi polysaccharides are complex carbohydrates found in the cell walls and extracellular matrices. These polysaccharides play a crucial role in the structural integrity and functionality of fungal organisms. They can be homopolysaccharides that contain repeating units of the same monosaccharide or heteropolysaccharides, containing several types of monosaccharides. Polysaccharides can also include other groups, such as sulfate or glucuronate, and adopt simple, triple, or random helical conformations depending on the conditions of the culture medium (Huang and Nie, 2015; Gong et al., 2020), significantly affecting their solubility in water or marginally basic aqueous solutions. Solubility and the type of conformation adopted are crucial for biological activity and must be controlled in different assays at the in vitro level because of their effects on the expected results. Structural conformation is also responsible for the great water solubility of polysaccharides: for example, scleroglucan secreted by Sclerotium fungi adopts a highly ordered, rigid, triple helical tertiary structure when dissolved in water at room temperature and low concentrations of alkali, usually below 0.15 M NaOH (Castillo et al., 2015). Water solubility of fungal exopolysaccharides (EPS) depends, to a large extent, on monosaccharide composition and structure and size of the respective molecules (Jaros et al., 2018). These variables, together with differences in the type of bonding and branching, tertiary structure, electrical charge and conformation, and solubility generate a diversity of physical and chemical properties, as well as reported biological activities (Bacic et al., 2009).
Polysaccharides produced by macrofungi can be classified into three main groups based on their location in the cell: (1) cytosolic polysaccharides that provide a source of carbon and energy for the cell; (2) cell wall polysaccharides that include the peptidoglycans, teichoic acids, and lipopolysaccharides; and (3) extracellular polysaccharides that are exuded in the form of capsules or biofilms, also known as EPS, which constantly diffuse into the cell wall and cell cultures (Kambourova et al., 2015; Wang et al., 2022). For the production of EPS, the submerged culture technique is used at both laboratory and industrial scales, where the mycelium of the fungus grows while floating as small spheres called pellets when there is agitation (Papagianni, 2004). The submerged fermentation process is widely used for producing different types of metabolites on a large scale or under flask conditions in which organisms can be obtained in limited physical spaces under conditions controlled and optimized for the production of biomass; another advantage is the ease of separating the mycelium from the EPS in the culture medium, obtaining a product of uniform quality for their purification in a further step. On the other hand, it provides several advantages such as higher productivity and yields, lower labor costs, and lower contamination risk. Submerged culture offers the potential advantages of faster production of EPS within a smaller space, reduced possibility of contamination, and better control of cultivation conditions such as carbon source and temperature (Dudekula et al., 2020).
In addition to submerged cultures, several other methods have been employed for the production of EPS. Some of these methods are: (1) surface culture, in which the microorganisms are grown on the surface of a solid substrate, such as agar or other suitable media; the EPS is produced and secreted by the microorganisms onto the surface of the substrate (Breitenbach et al., 2022); (2) solid-state fermentation, which involves the growth of microorganisms on solid substrates without the addition of free-flowing water; the microorganisms utilize the nutrients present in the solid substrate to produce EPS; common solid substrates used include agricultural residues, cereal grains, and sawdust (Isikhuemhen et al., 2014); (3) biofilm reactors, which provide an ideal environment for the growth of biofilms, allowing the microorganisms to attach to a surface and form a structured matrix; EPS production occurs with the biofilm structure (Seneviratne et al., 2008); (4) immobilized cell systems, in which the microbial cells are immobilized or attached to a solid support matrix, such as alginate beads, polyurethane foam, or glass beads; the immobilized cells produce EPS while attached to the support matrix, allowing for easy separation and recovery of the EPS (Li et al., 2017); and (5) membrane bioreactors, which combine the use of submerged culture with the retention of microbial cells and EPS using a membrane filtration system; the membrane allows the culture medium to pass through while retaining the microbial cells and EPS, which can then be harvested and recovered (Brito et al., 2019). It is important to note that the method of choice for EPS production depends on various factors, including the microorganism used, the type of EPS desired, and the scale of production. Different methods offer distinct advantages and disadvantages in terms of yield, productivity, cost, and ease of operation.
Extensive investigations have been conducted to enhance the yield of EPS production. These studies have focused on optimizing various factors, including the assessment of different carbon sources (e.g., glucose, sucrose, fructose) and nitrogen sources (e.g., peptone, yeast extract, ammonium nitrate). The influence of pH levels and incubation time on scleroglucan production has also been thoroughly examined. Notably, Fariña et al. (2001) successfully identified the optimal conditions for scleroglucan production by Sclerotium rolfsii. Their research determined the most advantageous carbon and nitrogen sources, optimal pH range, and incubation time, resulting in significant improvements in scleroglucan yields. Our approach differs from the method described by Fariña et al. (2001), as we evaluate the impact of two carbon sources. Moreover, we consider the contribution of the mushroom species as a crucial factor in EPS production. Incorporating a comprehensive range of tests during initial evaluation enables the identification of optimal strains for bioactive EPS production (Mahapatra and Banerjee, 2013; Angelova et al., 2022). Among agaricomycetes, Agaricales and Polyporales are the two most prominent and extensively used orders in biotechnology. To validate this protocol, representative strains from Agaricales and Polyporales were selected, namely Schizophyllum radiatum and Lentinus crinitus, respectively.
Materials and reagents
Petri dishes (90 mm × 15 mm) (Nest, catalog number: 752001)
Polypropylene microcentrifuge tubes (2.0 mL) (Eppendorf, Biologix, catalog number: 80-0020)
Paper towel
Conical tubes (50 mL) (Falcon, catalog number: 602002-1)
Erlenmeyer flask (250 mL)
Beakers (10 and 500 mL) (Glassco Aleman, catalog number: 229.202.02, 229.202.08A)
Borosilicate glass bottle with screw cap with rubber/Teflon liner (250 mL) (Glassco, catalog number: 6679.269.245.01)
Nichrome inoculating needle (Citoplus, catalog number: 33210001B)
Filter paper, pore size: 3 μm (Munktell Ahlstrom, catalog number: 391)
Filter paper, pore size: 10 μm (Ahlstrom Munksjo, catalog number: 389)
Pistil (Axygen, Pes15b)
Core samplers (diameter: 5 mm)
Magnetic stir bar (KartellTM, catalog number: 0076800)
Biological materials
Schizophyllum radiatum Fr. (Fungario Universidad del Tolima; Laboratory collection, strain 030, voucher: LRD27)
Lentinus crinitus (L.) Fr. (Fungario Universidad del Tolima; Laboratory collection, strain 147, voucher: ZF37)
Reagents
Agar agar (Sigma-Aldrich, catalog number: BCBS4444V, 05040)
Peptone (EMD, Millipore, catalog number: WM879031-906BD)
Yeast extract (OXOID, catalog number: LP0021)
Malt extract (OXOID, catalog number: LP0039)
D-Glucose (EMD Millipore, catalog number: K51794437 004)
D-Sucrose (Fisher Scientific, catalog number: S5-500)
Sodium hydroxide (NaOH) (EMD Millipore, catalog number: B1101198-436)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (Honeywell Specialty Chemicals Seelze, catalog number: 10314903)
Iron (II) sulfate heptahydrate (FeSO4·7H2O) (Sigma-Aldrich, catalog number: F7002)
Ammonium sulfate [(NH4)2SO4] (PanReac Química SLU, catalog number: A1032)
Anhydrous dipotassium hydrogen phosphate (K2HPO4) (Mallinckrodt Baker, catalog number: 3252-01)
Ethanol, 96% (Fábrica de Licores Tolima)
Solutions
Malt agar medium (see Recipes)
SYAMI culture medium (see Recipes)
Sodium hydroxide (NaOH) 0.1 N (see Recipes)
Recipes
Malt agar medium
Reagent Final concentration Peptone 3 g/L Malt extract 30 g/L Agar-agar 15 g/L SYAMI culture medium
Reagent Final concentration MgSO4·7H2O 0.05 g/L K2HPO4 2.0 g/L FeSO4·7H2O 0.2 g/L (NH4)2SO4 0.15 g/L Yeast extract 2.0 g/L Carbon source (glucose or sucrose) 40 g/L Sodium hydroxide (NaOH) 0.1 N
Reagent Quantity NaOH 1 g H2O 250 mL
Equipment
Centrifuge (HERMLE Labortechnik GmbH, Z326K, catalog number: 311)
Orbital shaker (IKA LABORTECHNIK, KS250 basic)
Fume hood (Jpinglobal, catalog number: JPCEGH1200-BB-PP)
Laminar flow cabinet, horizontal (Purificación y análisis de fluidos)
Ultra-low temperature freezer, -85 °C (Kaltis, catalog number: 390)
Freeze dryer (Buchi, model: Lyovapor L-200)
Autoclave (All American, model: 1941X)
Refrigerator (COLDLINE, model: FORTE V13-RHC)
Barnstead Smart2Pure UV/UF (Thermo Scientific, type: Smart2Pure 3 UV/UF)
Hot plate magnetic stirrer (Stuart, catalog number: UC152)
Analytical balance (KERN, type: ACS 220-4)
Blender (Recco, model: 242748)
Drying stove (Memmert, catalog number: ref 1470)
Desiccator (Metacrilato 250 mm)
Sieve (Endecotts Ltd., ASTM E-11, catalog number: 65803- TAN AC8-1-1/2, 37,3)
Borosilicate glass filtration system (Glassco, catalog number: COL-764-260.202.01)
Incubator, 28 °C (Binder, catalog number: BD023UL)
pH meter (SI Analytics, type: HandyLab 100)
Water bath (Buchi, catalog number: B-480)
Software and datasets
Microsoft Excel for calculations
RStudio Team, 2015. RStudio: Integrated Development Environment for R. Boston, MA. Available at: http://www.rstudio.com/
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Dávila Giraldo, L. R., Villanueva Baez, P. X., Zambrano Forero, C. J. and Arango, W. M. (2023). Production, Extraction, and Solubilization of Exopolysaccharides Using Submerged Cultures of Agaricomycetes. Bio-protocol 13(19): e4841. DOI: 10.21769/BioProtoc.4841.
分类
生物科学 > 生物技术
微生物学 > 微生物生物化学 > 糖类
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













