- EN - English
- CN - 中文
Fluorescence Resonance Energy Transfer to Detect Plasma Membrane Perturbations in Giant Plasma Membrane Vesicles
荧光共振能量转移检测巨型质膜囊泡中的质膜扰动
(*contributed equally to this work) 发布: 2023年10月05日第13卷第19期 DOI: 10.21769/BioProtoc.4838 浏览次数: 2059
评审: Alessandro DidonnaAnonymous reviewer(s)
Abstract
Disruptions and perturbations of the cellular plasma membrane by peptides have garnered significant interest in the elucidation of biological phenomena. Typically, these complex processes are studied using liposomes as model membranes—either by encapsulating a fluorescent dye or by other spectroscopic approaches, such as nuclear magnetic resonance. Despite incorporating physiologically relevant lipids, no synthetic model truly recapitulates the full complexity and molecular diversity of the plasma membrane. Here, biologically representative membrane models, giant plasma membrane vesicles (GPMVs), are prepared from eukaryotic cells by inducing a budding event with a chemical stressor. The GPMVs are then isolated, and bilayers are labelled with fluorescent lipophilic tracers and incubated in a microplate with a membrane-active peptide. As the membranes become damaged and/or aggregate, the resulting fluorescence resonance energy transfer (FRET) between the two tracers increases and is measured periodically in a microplate. This approach offers a particularly useful way to detect perturbations when the membrane complexity is an important variable to consider. Additionally, it provides a way to kinetically detect damage to the plasma membrane, which can be correlated with the kinetics of peptide self-assembly or structural rearrangements.
Key features
• Allows testing of various peptide–membrane interaction conditions (peptide:phospholipid ratio, ionic strength, buffer, etc.) at once.
• Uses intact plasma membrane vesicles that can be prepared from a variety of cell lines.
• Can offer comparable throughput as with traditional synthetic lipid models (e.g., dye-encapsulated liposomes).
Graphical overview
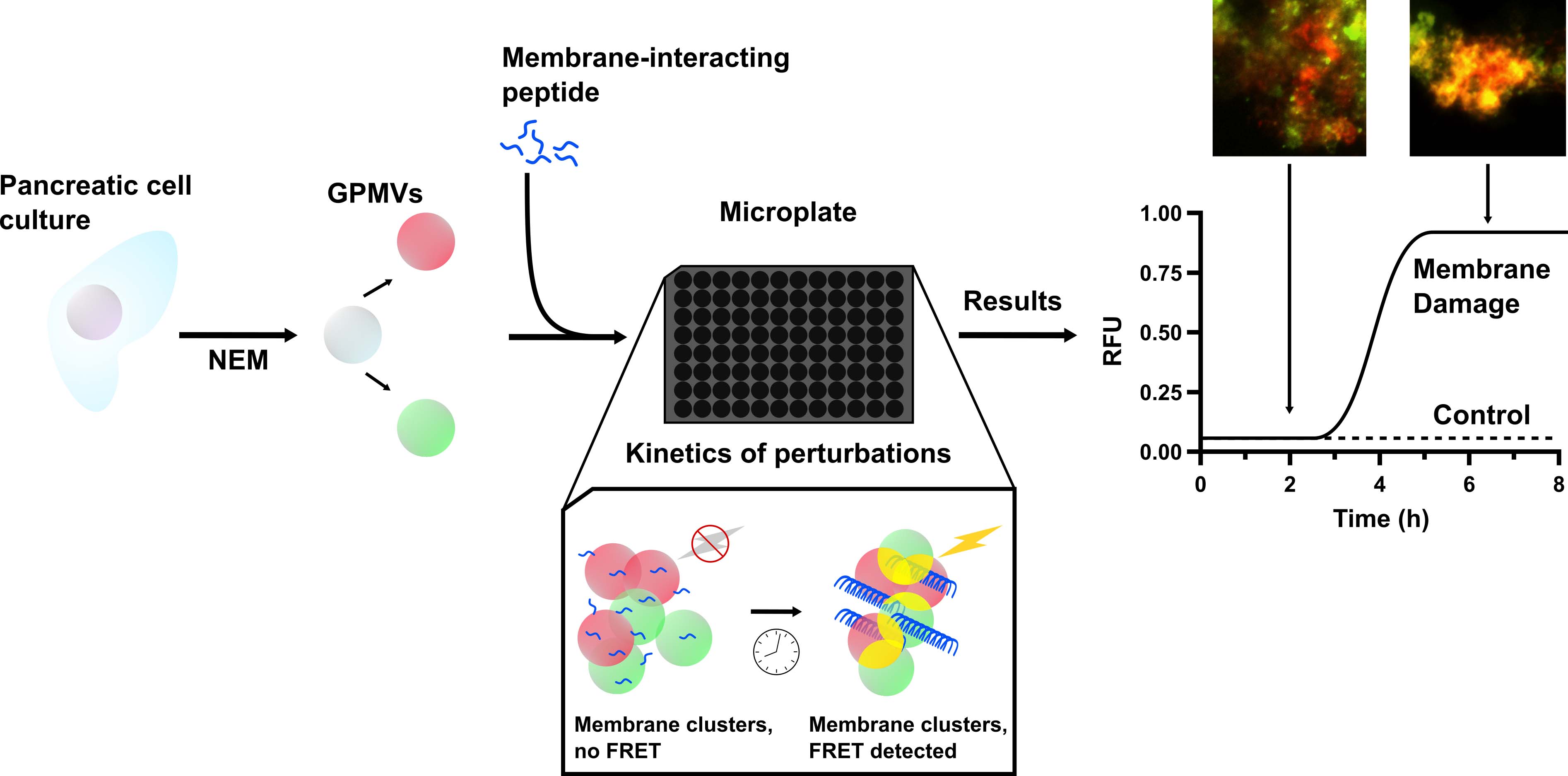
Background
The plasma membrane is a highly dynamic, heterogenous barrier between the intracellular components and the extracellular space ( Harayama and Riezman, 2018; Kalappurakkal et al., 2020). The integrity of this barrier is paramount to maintaining normal cell function and, consequently, a great deal of importance is attributed to understanding the processes by which it is disturbed. In particular, numerous amyloidogenic proteins have been found to interact with and destabilise the plasma membrane (Sciacca et al., 2018). While the biophysics of membrane disruption during amyloid formation are typically studied using synthetic models (e.g., liposomes, lipid monolayers, black lipid membranes), these models fall short of accurately depicting the complexity of plasma membranes (Thakur et al., 2011; Serra-Batiste et al., 2016; Sciacca et al., 2020). In vivo, plasma membranes are composed of not only lipids but also a significant amount of proteins (approximately 50% w/w) and polysaccharides (Kusumi et al., 2012; Harayama and Riezman, 2018). This heterogeneity is difficult, if not impossible, to recreate in a synthetic model, and it is highly probable that the molecular composition of the model system can influence protein aggregation and subsequent disruption of the membrane (Zhang et al., 2017). Prior work has demonstrated that varying the proportions of anionic phospholipids, cholesterol, and gangliosides has dramatic effects on the permeability of liposomes (Song et al., 2014; Sciacca et al., 2016 and 2020). Similarly, an accurate prediction of the cytotoxicity based on membrane damage observed in synthetic models alone remains challenging (Cao et al., 2013).
In several recent studies, giant plasma membrane vesicles (GPMVs) were employed to investigate the biophysics of amyloid-associated membrane damage (Quittot et al., 2021; Birol et al., 2018; Schlamadinger and Miranker, 2014). GPMVs are useful models isolated from different cells, such as HeLa, HepG2, Caco-2, RBL, CHO, or INS using comparable and straightforward protocols (Sezgin et al., 2012; Zartner et al., 2021). Consequently, membrane composition of GPMVs is virtually indistinguishable from the parent cells (K.R. Levental and Levental, 2015), although it must be noted that the relative distribution between the inner and outer phospholipid leaflets—in particular those containing anionic phosphatidylserine head groups—can be altered to a non-negligible extent in the vesicle membranes (Sezgin et al., 2012). These recent approaches are limited, however, in their ability to monitor large numbers of samples in a high-throughput fashion. The protocol described here compensates for the current shortcomings of GPMV-based analyses through the use of a microplate assay, measuring the Förster resonance energy transfer (FRET) between two differently labelled vesicles in a suspension. This allows for an experimental throughput comparable to that of traditional liposomes, such that an entire 96-well microplate can be analysed in a single experiment. While this assay is relatively robust and can detect changes to membranes over time, it is limited in its inability to detect small-scale perturbations. Due to the nature of the FRET response, membranes must be in close proximity and under somewhat static conditions. Additionally, smaller defects such as the formation of transmembrane pores may not be detectable with this method. If evaluating transmembrane pore formation with GPMVs is desired, an elegant protocol was developed by Birolet al. (2018), in which cytoplasmic proteins are fluorescently labelled and whose release can be monitored (Birol et al., 2018). Nonetheless, the following approach proves itself to be a valuable source of biophysical data regarding the relative degree of membrane damage during amyloid formation. Additionally, this approach may also be applicable to other membrane-damaging compounds such as antimicrobial and lytic peptides.
Materials and reagents
Biological materials
Rat INS-1E pancreatic cells (provided as a donation by Dr. Marc Prentki at the Centre hospitalier de l’Université de Montréal. Cells can be sourced from Accegen, catalog number: ABC-TC233S)
Reagents
N-ethylmaleimide (NEM) (Sigma-Aldrich, catalog number: E3876)
Human islet amyloid polypeptide (IAPP) (synthesised in-house, can be ordered from Genscript, catalog number: RP11278)
KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY-NH2
Note: Disulfide bridge between C2–C7.
Rat islet amyloid polypeptide (rIAPP) (synthesised in-house, can be ordered from Genscript, catalog number: RP11280)
KCNTATCATQRLANFLVRSSNNLGPVLPPTNVGSNTY-NH2
Note: Disulfide bridge between C2–C7.
Ammonium molybdate (Sigma-Aldrich, catalog number: 277908)
Ascorbic acid (Sigma-Aldrich, catalog number: AX1775)
Phosphorus standard (Sigma-Aldrich, catalog number: P3869)
Sulfuric acid, 98% (H2SO4) (Sigma-Aldrich, catalog number: 258105))
Hydrogen peroxide, 30% (H2O2) (Thermo Fisher, catalog number: 033323-AP)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
Sodium hydroxide (NaOH) (Sigma Aldrich, catalog number: 221465)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C4901)
HEPES, free acid (Sigma-Aldrich, catalog number: 391314)
HEPES, 1 M solution (Cytiva, catalog number: SH30237.01)
Sodium pyruvate (Cytiva, catalog number: SH30239.01)
Penicillin-Streptomycin, 100× (Cytiva, catalog number: SV30010)
Fetal bovine serum (FBS) (Cytiva, catalog number: SH30070.03)
RPMI 1640, with L-glutamine (Cytiva, catalog number: SH30027.01)
Hank’s buffered saline solution (HBSS), without calcium, magnesium, and phenol red (Cytiva, catalog number: SH30588.01)
Trypsin 1× with 0.05% EDTA (Wisent, catalog number: 325-042-CL)
β-mercaptoethanol (Sigma-Aldrich, catalog number: M3148)
FAST-DiO (Invitrogen, Thermo Fisher, catalog number: D3898)
FAST-DiI (Invitrogen, Thermo Fisher, catalog number: D7756)
Wash buffer (see Recipes)
1 M sodium hydroxide (NaOH) (see Recipes)
Giant plasma membrane vesicle (GPMV) buffer (see Recipes)
3.5% m/v ammonium molybdate (IV) (see Recipes)
10% m/v ascorbic acid (see Recipes)
Complete growth media (see Recipes)
8.9 N H2SO4 (see Recipes)
1 mg/mL FAST-DiO (see Recipes)
1 mg/mL FAST-DiI (see Recipes)
Recipes
Wash buffer
10 mM HEPES, 150 mM NaCl, 2 mM CaCl2, pH 7.4
In a beaker, add 400 mL of ultrapure (type 1) water.
Dissolve HEPES (1.192 g), NaCl (4.38 g), and CaCl2 (0.111 g).
Using a pH meter, slowly add 1 M NaOH under stirring to bring the pH to 7.4.
Transfer the solution to a 500 mL volumetric flask and add ultrapure (type 1) water to complete the volume.
This solution must be filtered through a 0.2 μm PES bottle filter inside of a laminar flood hood before use.
Store the bottle at 4 °C.
1 M NaOH
Weigh out 4 g of NaOH pellets.
In a beaker, add 75 mL of ultrapure (type 1) water.
Slowly add the NaOH pellets to the water while stirring until completely dissolved.
Transfer the solution to a 100 mL volumetric flask and add more ultrapure (type 1) water to complete the volume.
This solution can be stored in a brown glass bottle at room temperature.
CAUTION: The dissolution of NaOH is highly exothermic. This must be done slowly to avoid boiling. For larger volumes, consider keeping the mixing beaker on ice to avoid excessive heat generation.
GPMV buffer
10 mM HEPES, 150 mM NaCl, 2 mM CaCl2, 2 mM NEM, pH 7.4
In a 50 mL conical tube, add 12.5 mg of NEM.
Fill to the 50 mL line with wash buffer.
Once dissolved, filter this solution through a 0.22 μm syringe tip filter inside of a laminar flow hood before use.
Note: This solution must be prepared fresh each time GPMVs are to be produced, as the NEM is not stable in solution for prolonged duration.
3.5% m/v ammonium molybdate (IV)
In a 100 mL volumetric flask, add 3.5 g of ammonium molybdate (IV).
Add 50 mL of ultrapure (type 1) water and ensure that the solid is completely dissolved.
Fill to the 100 mL line with ultrapure (type 1) water.
Transfer to a brown glass bottle and store at 4 °C.
10% m/v ascorbic acid
In a 100 mL volumetric flask, add 10.0 g of ascorbic acid.
Add 50 mL of nanopure water and ensure that the solid is completely dissolved.
Fill the rest to the 100 mL line with nanopure water.
Transfer to a brown glass bottle and store at 4 °C.
Complete growth media
RPMI 1640, 100 U/mL pen-strep, 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate, 50 mM β-mercaptoethanol
Inside of a laminar flow hood, add the following to a 500 mL bottle of RPMI 1640:
5 mL of penicillin-streptomycin (100×).
50 mL of FBS.
5 mL of HEPES (1 M).
5 mL of sodium pyruvate (100 mM).
1.75 μL of β-mercaptoethanol. CAUTION: β-mercaptoethanol is strongly odorous. Ensure adequate ventilation before using this product.
Store this medium at 4 °C.
Note: Working under aseptic conditions is essential here to avoid contaminating the cell culture.
8.9 N H2SO4
Measure 75.8 mL of nanopure water into a glass bottle.
Slowly add 24.2 mL of concentrated (95%–98%) H2SO4. CAUTION: The dilution of H2SO4 is exothermic and should be done with care. Keep the solution on ice and under constant stirring while slowly adding the acid.
1 mg/mL FAST-DiO
Dissolve 1.0 mg of FAST-DiO with 1.0 mL of DMSO.
Prepare aliquots of this master solution by dispensing 50 μL into separate 0.5 mL microfuge tubes.
Note: This compound is light sensitive, particularly when not incorporated into a lipid bilayer. Minimise exposure to ambient light. Using smaller aliquots minimises the number of freeze/thaw cycles for each aliquot.
1 mg/mL FAST-DiI
Dissolve 1.0 mg of FAST-DiO with 1.0 mL of DMSO.
Prepare aliquots of this master solution by dispensing 50 μL into separate 0.5 mL microfuge tubes.
Note: This compound is light sensitive, particularly when not incorporated into a lipid bilayer. Minimise exposure to ambient light. Using smaller aliquots minimises the number of freeze/thaw cycles for each aliquot.
Laboratory supplies
96-well microplates, black, non-binding surface with clear bottom (Corning, catalog number: 3651)
Silicone microplate covers (Corning, catalog number: 3090)
100 mm tissue culture dishes (Sarstedt, catalog number: 3902)
150 mm tissue culture dishes (Sarstedt, catalog number: 3903)
2 mL serological pipettes (Sarstedt, catalog number: 1252025)
5 mL serological pipettes (Sarstedt, catalog number: 1253025)
10 mL serological pipettes (Sarstedt, catalog number: 1254025)
25 mL serological pipettes (Sarstedt, catalog number: 1685020)
15 mL conical centrifuge tubes (Sarstedt, catalog number: 62.554.100)
50 mL conical centrifuge tubes (Sarstedt, catalog number: 62.547.004)
0.5 mL microfuge tubes (Sarstedt, catalog number: 72.704)
100 kDa centrifugal filter units (Amicon, Millipore, catalog number: UFC910024)
0.2 μm PES bottle filter units (ThermoFisher, catalog number: 569-0020)
10 mL syringes (BD, catalog number: 309604)
0.22 μm syringe filters (Sarstedt, catalog number: 83.1826.001)
16 mm × 150 mm glass reaction tubes (VWR, catalog number: 47729-580)
12 mm × 75 mm glass reaction tubes (VWR, catalog number: 47729-570)
Plastic plugs for 12 mm × 75 mm reaction tubes (VWR, catalog number: 60819-003)
Brown glass bottles (Sigma-Aldrich, catalog number: DWK218062454)
Microplate lids with silicone seal (Corning, catalog number: 07-200-699)
18 G needles (BD precision glide, catalog number: 305195)
Equipment
Plate reader (Molecular Devices, Spectramax i3)
Centrifuge (Thermo, IEC CL30) with swinging bucket rotor (S41*)
Laminar flow hood (VWR Microzone)
Incubator (Thermo, Heracell 150i)
Hot plate (Fisherbrand, Isotemp)
Aluminium heating block (Thermo Fisher, catalog number: 88880136)
pH meter (Fisherbrand, Accumet AE150)
Micropipettes (Gilson Pipetteman P200, catalog number: F144058M)
Vortex mixer (Scientific industries vortex genie-2, catalog number: SI-0236)
Metal bowl or glass beaker, large enough to contain a test tube rack and capable of being heated at 100 °C. Can be replaced for a water bath if one is available
Software and datasets
Prism 8 (GraphPad)
Excel, Office 16 (Microsoft)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Sebastiao, M., Quittot, N., Marcotte, I. and Bourgault, S. (2023). Fluorescence Resonance Energy Transfer to Detect Plasma Membrane Perturbations in Giant Plasma Membrane Vesicles. Bio-protocol 13(19): e4838. DOI: 10.21769/BioProtoc.4838.
分类
细胞生物学 > 细胞结构 > 细胞质膜
生物化学 > 脂质 > 膜脂
生物物理学 >
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link