- EN - English
- CN - 中文
Application of Electrical Stimulation to Enhance Axon Regeneration Following Peripheral Nerve Injury
电刺激增强周围神经损伤后的轴突再生的应用
发布: 2023年10月05日第13卷第19期 DOI: 10.21769/BioProtoc.4833 浏览次数: 1988
评审: Alessandro DidonnaAnand Ramesh PatwardhanZheng Zachory Wei
Abstract
Enhancing axon regeneration is a major focus of peripheral nerve injury research. Although peripheral axons possess a limited ability to regenerate, their functional recovery is very poor. Various activity-based therapies like exercise, optical stimulation, and electrical stimulation as well as pharmacologic treatments can enhance spontaneous axon regeneration. In this protocol, we use a custom-built cuff to electrically stimulate the whole sciatic nerve for an hour prior to transection and repair. We used a Thy-1-YFP-H mouse to visualize regenerating axon profiles. We compared the regeneration of axons from nerves that were electrically stimulated to nerves that were not stimulated (untreated). Electrically stimulated nerves had longer axon growth than the untreated nerves. We detail how variations of this method can be used to measure acute axon growth.
Keywords: Peripheral nerve injury (周围神经损伤)Background
Peripheral nerve injury is an important clinical problem; generally, the functional recovery is very poor (Lee and Wolfe, 2000; Höke and Brushart, 2010; Enax-Krumova et al., 2017; Bulut et al., 2018; Zheng et al., 2018; Wang et al., 2019) if the injury is more proximal to the spinal cord, involves a gap of ≥ 5 mm, or involves mixed sensory and motor nerves. The main reason for this poor recovery is that the injured neurons are required to regenerate their axons over long distances, sometimes many centimeters, to reconnect to their peripheral target. The regenerating axons have a very slow rate of growth of ∼1 mm/day (Sulaiman and Gordon, 2000 and 2013; Sulaiman and Kline, 2006; Sulaiman et al., 2011). Our lab and many others have demonstrated that activity-based therapies (like exercise in the form of treadmill running, optical stimulation, or electrical stimulation) after peripheral nerve injury significantly enhances axon regeneration (Al-Majed et al., 2000; Brushart et al., 2002; Wood et al., 2012; Thompson et al., 2014; Gordon and English, 2016; Ward and English, 2019). Many studies have shown that electrical stimulation for 1 h at 20 Hz enhances axon regeneration after nerve crush or transection models in rats, mice (Al-Majed et al., 2000; Brushart et al., 2002 and 2005; English et al., 2007; Geremia et al., 2007; Elzinga et al., 2015; Shapira et al., 2019), and human subjects (Gordon et al., 2010; Wong et al., 2015; Barber et al., 2018). More recently, conditioning electrical stimulation of the intact nerve, prior to nerve transection and repair, has been shown to enhance nerve regeneration and functional recovery (Senger et al., 2018, 2019, 2020a and 2020b; Gordon, 2020). Here, we demonstrate that a single session of electrical stimulation for an hour at a controlled frequency applied to the nerve using a cuff prior to transection and repair significantly enhances axon regeneration compared to no treatment. We used Thy-1-YFP-H mice (Jackson Laboratory, stock no. 003782) to visualize regenerating axons. Thy-1-YFP-H are transgenic mice that express yellow fluorescent protein (YFP) at high levels in a subset of motor and sensory neurons, as well as subsets of central neurons. The axons are brilliantly fluorescent all the way to the terminals, which provides a useful visual outcome that can be quantified to compare regeneration. The H strain is nearly identical to Thy-1-YFP-16 (Jackson laboratory, stock no. 003709), except for the percentage of neurons expressing YFP. Thy-1-YFP-H express YFP in 10%–30% of dorsal root ganglia sensory neurons, whereas expression of Thy-1-YFP-16 is much greater. The advantage of sparse labeling is that individual axons can be accurately and precisely (length and branching) traced into the distal stump. The advantage of using an electrical cuff to stimulate (in contrast to needle electrodes or wires) is that the target nerve can be selectively stimulated without unwanted stimulation of muscle or neighboring nerves. The sciatic nerve is commonly used to study peripheral axon regeneration, and it consists of three terminal branches (Figure 1) that are held together by epineurium to form the whole sciatic nerve. The epineurium can be delicately surgically removed to separate the branches proximally, and the cuff can be placed on specific branches of the sciatic nerve for various experimental designs.
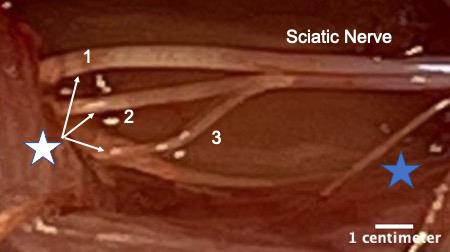
Figure 1. White star shows the three terminal branches of the sciatic nerve. 1) Tibial nerve, 2) common fibular or common peroneal nerve, and 3) sural nerve. The branches were separated by removing a portion of the epineurium using fine forceps. Blue star indicates a small proximal branch of the sciatic nerve that innervates a portion of the hamstring muscle. This image was taken on an AmScope ZM-4TW3-FOR-9M Digital Professional Trinocular Stereo Zoom Microscope at 4.5× zoom.
Materials and reagents
Silastic sheeting (Dow Corning Corporation medical products, catalog number: 501-1)
0.5 mL tubes (Corning, catalog number: 3750)
Thrombin (MP Biomedical, catalog number: 76461-568)
Fibrinogen (Sigma, catalog number: F3879-1G)
Fibrin glue (Akhter et al., 2019; Ward and English, 2019)
Isoflurane (Piramal Healthcare, catalog number: RXISO-250)
Gauze pads (Fisher Brand, catalog number: 22-362-186)
Betadine solution (Purdue Frederick Company, catalog number: 10224)
Ethanol (Thermo Fisher, catalog number: T038181000CS)
10 μL pipette tips (Eppendorf, catalog number: 022492004)
0.9% sodium chloride (NaCl) (Fisher, catalog number: S271-1)
4% PFA (paraformaldehyde prepared in 0.1 M PBS) (Sigma, catalog number: P6148-500G)
1× phosphate buffered saline (PBS) prepared from PBS tablets (Sigma, catalog number: P4417-100TAB)
Euthanasia solution (10 mg/mL made in sterile saline) (Med Vet International, catalog number: RXEUTHASOL)
Sylgard 184 silicone elastomer base and curing agent (Krayden DOW, catalog DC4019862)
Equipment
Cuff electrode
Dumont Forceps #5 (Fine Science Tools, catalog number: 11251-10)
Surgical scissors (Fine Science Tools, catalog number: 14084-09)
Spring scissors (Fine Science Tools, catalog number: 15006-09)
Silastic tubing (Laboratory Tubing, catalog number: 508-006)
Insulated wire (Cooner Wire, catalog number: AS631)
Flexible silicone (Dow Corning, catalog number: Sylgard® 184)
Sewing needle size #9 (Singer, catalog number: 01125)
Table clamp (to hold the silicone tubing in place)
Wooden applicator stick (Electron microscopy sciences, catalog number: 07230)
Silk braided thread size 0 (J.A Daknatel & Son Inc., catalog number: 3-766-900)
Microscope (Nikon, model number: SMZ800)
LED Tester, set at 15 V for checking current leakage from cuff electrode (Hewlett Packard Palo Alto Hp California 630)
For electrical stimulation
Grass Stimulator and Amplifier Box (Ward and English, 2019)
Custom Lab View Program (Ward and English, 2019)
Surgery
Thy-1 YFP-H adult mice (2–4 months old; male and/or female weighing 18–30 g) (Jax laboratory, stock number: 003782)
Surgical drapes (Med-Vet International, catalog number: SKU DR1826)
Needle holder with suture cutter (Fine Science Tools, catalog number: 12502-14)
Hair clipper (Wahl combo kit #9990-1201)
Deltaphase Isothermal Pad (Braintree scientific, model: 39DP)
Warm water circulator (Gaymar TP500)
Surgical stereoscope (AmScope SM-4BZ-80S)
Glass bead sterilizer (Braintree Scientific, catalog number: GER 5287-120V)
Surgical board (Fisher Brand, catalog number: 0900224C)
Surgical tape (3M Micropore tape, 1532-1)
Cotton tipped applicator (Dukal corporation, 9006)
Eye ointment (Refresh P.M.)
Triple antibiotic ointment (Mckesson Medical Surgical Inc, catalog number: 955410)
Meloxicam oral suspension (Med-Vet International, catalog number: RXMELOXIDYL10)
Absorbable: coated Visorb undyed braided polyglycolic acid suture NSF-2 19 mm 3/8 30" (75 cm) (CP medical, catalog number: 421A)
Non-absorbable Monofilament polyamide suture (Redilon 5-0) 1 metric 18" (45 cm) (CP medical, catalog number: 661B)
Software
FIJI (NIH) to process and analyze the images
Microsoft Excel to organize the axon length data
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wariyar, S. S. and Ward, P. J. (2023). Application of Electrical Stimulation to Enhance Axon Regeneration Following Peripheral Nerve Injury. Bio-protocol 13(19): e4833. DOI: 10.21769/BioProtoc.4833.
分类
神经科学 > 周围神经系统 > 坐骨神经
神经科学 > 神经解剖学和神经环路 > 坐骨神经
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link