- EN - English
- CN - 中文
Recoil Measurements in Drosophila Embryos: from Mounting to Image Analysis
果蝇胚胎的反冲测量:从安装到图像分析
发布: 2023年07月20日第13卷第14期 DOI: 10.21769/BioProtoc.4806 浏览次数: 1455
评审: Alberto RissoneKai YuanAnonymous reviewer(s)
Abstract
Tension and force propagation play a central role in tissue morphogenesis, as they enable sub- and supra-cellular shape changes required for the generation of new structures. Force is often generated by the cytoskeleton, which forms complex meshworks that reach cell–cell or cell–extracellular matrix junctions to induce cellular rearrangements. These mechanical properties can be measured through laser microdissection, which concentrates energy in the tissue of interest, disrupting its cytoskeleton. If the tissue is undergoing tension, this cut will induce a recoil in the surrounding regions of the cut. This protocol describes how one can perform laser microdissection experiments and subsequently measure the recoil speed of the sample of interest. While we explain how to carry out these experiments in Drosophila embryos, the recoil calibration and downstream analyses can be applied to other types of preparations.
Key features
• Allows measuring tension in live Drosophila embryos with a relatively simple approach.
• Describes a quick way to mount a high number of embryos.
• Includes a segmentation-free recoil quantification that reduces bias and speeds up analysis.
Graphical overview
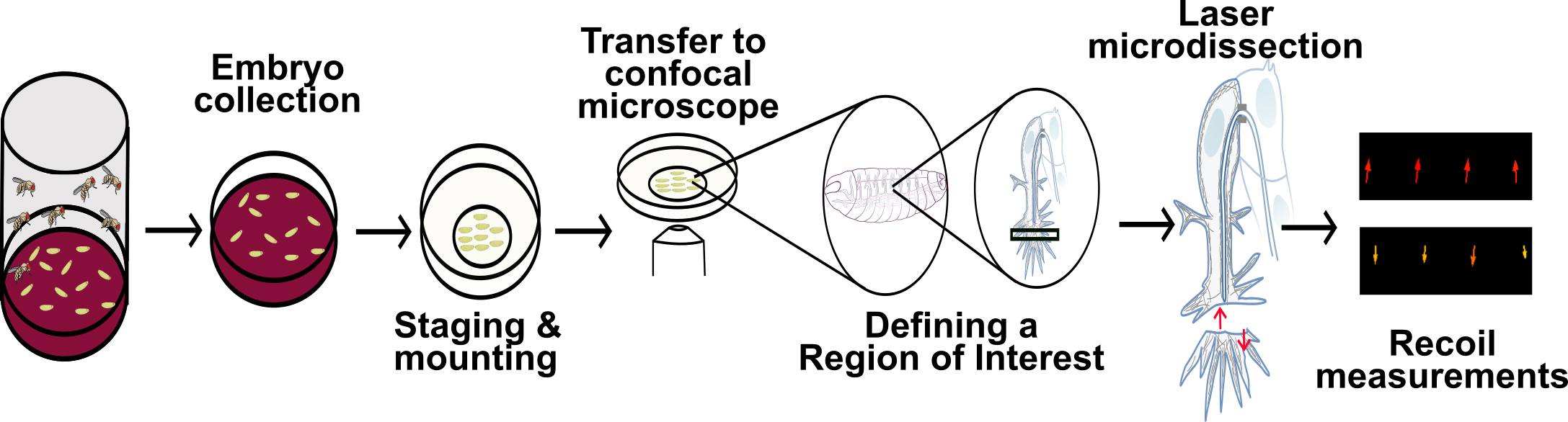
Background
Tissue morphogenesis requires that cells change their individual shapes and rearrange with one another to form novel structures. Most often, these processes depend on tension and force propagation to induce cell shape changes (Barrera-Velázquez and Ríos-Barrera, 2021; Inman and Smutny, 2021). Forces can act within the same cell or specific subcellular compartments, but can also act in neighboring cells with different biochemical and mechanical properties (Bhide et al., 2021; Ríos-Barrera and Leptin, 2022).
Cytoskeletal elements like actin are often the molecular basis of force generation. Actin fibers interact with motors and crosslinkers to generate force that induces cell autonomous and non-autonomous shape changes (Belmonte et al., 2017; Miao and Blankenship, 2020). Nevertheless, the sole presence of actin meshworks within a cell or tissue may not be indicative of tissue tension. To show that a given structure is under tension, one must manipulate or measure the mechanical properties of the tissue of interest. Different tools have been developed to do this, with particular advantages and caveats. Optogenetics (Izquierdo et al., 2018; Krueger et al., 2019) has a high temporal and spatial specificity but requires the incorporation of genetic tools that are sensitive to light, requiring special handling conditions and restricting imaging possibilities. Atomic force microscopy (Haase and Pelling, 2015) provides a direct readout of tissue properties but uses dedicated equipment and only works in direct contact with the specimen of interest. Fluorescent tension sensors (Lemke et al., 2019) do not require external manipulations but depend on complex image analyses to obtain quantitative information. Laser microdissection was among the first tools that enabled probing mechanical properties of tissues. It consists in focusing high-frequency photons within a region of interest (ROI), which will then disrupt and destabilize actin fibers (Nishimura et al., 2006). Severing the actin cytoskeleton using laser microdissection alters the force distribution in the cell or tissue of interest, resulting in a recoil in the direction in which the tissue is being stretched. Initial recoil speed of the tissue is directly proportional to the tension of the sample immediately before the laser procedure (Rauzi and Lenne, 2014; Shivakumar and Lenne, 2016). Therefore, by measuring the recoil speed, it is possible to infer the tension exerted in the sample of interest. This provides a quantitative approximation of the forces participating in the process of interest and can be used to compare tension generation in different directions (anisotropy), in different regions and stages of embryo development, or in varying genetic conditions (genetic gain- and loss-of-function studies).
There are several ways in which one can measure recoil speed [for an alternative approach, see Bio-protocol by Liang et al. (2016)]. In this protocol, we use particle image velocimetry (PIV) analyses, a straightforward approach that does not rely on image segmentation or manual annotation of the signal of interest (Tseng et al., 2012). Instead, PIV compares the spatial distribution of the fluorescent signal within two consecutive time points by comparing the signal within small interrogation windows within the ROI. A shift in the signal in an interrogation window is converted into a vector, which depicts the strength and direction of recoil, if there is any.
As an example of this procedure, we use tracheal terminal cells of the Drosophila respiratory system, which uses actin to coordinate their direction of migration with the formation of a subcellular tube (Ríos-Barrera and Leptin, 2022). Nevertheless, this approach can be used to study a wide range of developmental processes in the fruit fly and in other model systems.
Materials and reagents
Strains of Drosophila melanogaster expressing the actin-binding domain of Utrophin fused to GFP under the control of a UAS promoter (Flybase ID FBtp0073094) or the ubiquitously-expressed sqh promoter [Flybase ID FBtp0073095 (Rauzi et al., 2010; Bhide et al., 2021; Ríos-Barrera and Leptin, 2022)].
Paintbrush #3, round (commercially available)
Scalpel blade, #23 (e.g., EMS, catalog number: 72049-23)
Wash bottle containing distilled water (e.g., VWR, catalog number: 215-4306)
Forceps Dumont no. 5 (Merck, catalog number: F6521-1EA)
Commercial bleach 50% in water (commercially available)
35 mm glass-bottom dish plates (MatTek, catalog number: P35G-1.5-7-C) or plastic dish plates compatible with confocal microscopy (SPL Life sciences, catalog number: 210350)
Halocarbon oil 27 (Sigma-Aldrich, catalog number: H8773-100ML)
Heptane glue (see Recipes)
200 mL heptane (Sigma-Aldrich, catalog number: H2198-1L)
3M cellophane, double-sided tape (commercially available)
Embryo collection plates (see Recipes, Figure 1A)
6 cm Petri dishes (Sigma-Aldrich, catalog number: P5481-500EA)
250 mL distilled water
10 g agar (Millipore, catalog number: 9002-18-0)
4.2 g sugar molasses or brown sugar (commercially available)
250 mL apple juice (commercially available)
5 mL 10% Nipagin (Merck, catalog number: H5501-100G) in EtOH (Sigma-Aldrich, catalog number: 64-17-5)
Dried yeast (commercially available)
100 mL plastic beakers (e.g., Sigma-Aldrich, catalog number: Z245380)
Embryo basket (see Recipes, Figure 1B and 1C)
50 mL conical tube (e.g., Corning, catalog number: CLS430828)
Stainless steel mesh, 100 wires per inch (e.g., Amazon, Brand LTKJ)
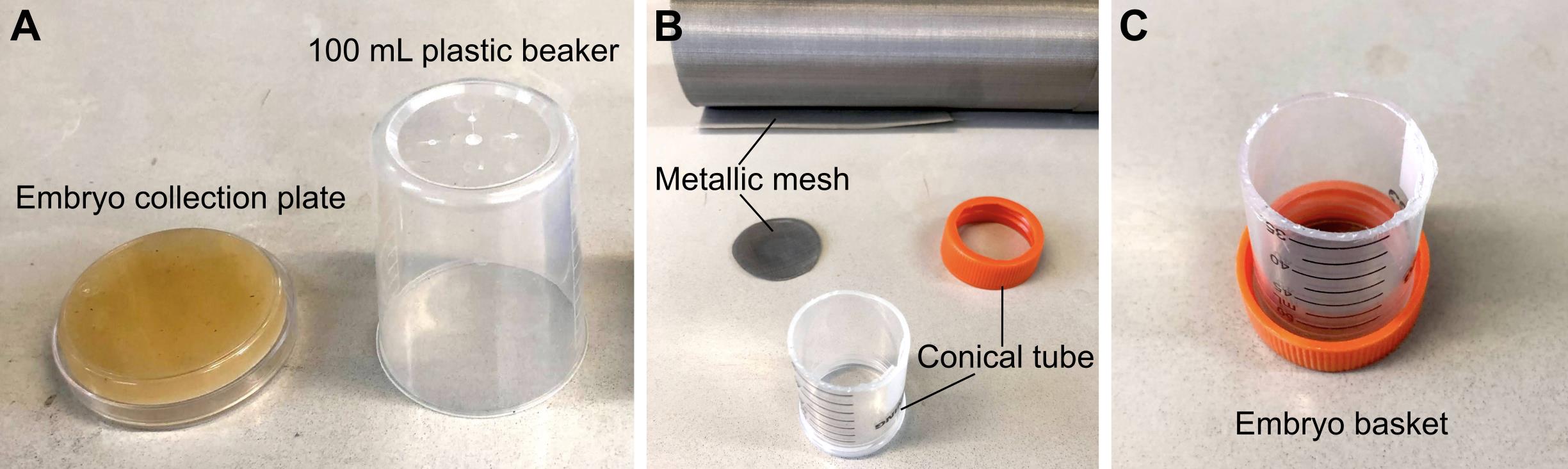
Figure 1. Materials required for embryo collection. (A) 6 cm embryo collection plates and 100 mL plastic beakers are combined to make embryo collection cages. (B) Materials required to make an embryo basket. (C) Assembled embryo basket.
Recipes
Heptane glue
Mix the tape and heptane for 3 h in a rocker.
Discard the tape and use the liquid for downstream applications.
Store the heptane glue in a dropper bottle.
Embryo collection plates
Dissolve the agar and brown sugar in 250 mL of water and autoclave.
Add 250 mL of apple juice, mix, and add the Nipagin solution.
Pour into 6 cm Petri dishes and store at 4 °C.
Before using, add yeast paste (dried yeast dissolved in water).
Punch small holes on the base of the plastic beakers. These will contain the flies, and the Petri dish with yeast will serve as a lid of the embryo collection cage.
Embryo basket
Make an open-ended 5 cm cylinder by cutting the conical tube using a cutter knife.
Cut a round window on the lid of the conical tube.
Cut the metallic mesh into a circle that fits inside the lid.
Screw in the lid containing the metallic mesh back into the conical tube.
Dissecting microscope (e.g., Velab, VE-S7)
Laser scanning confocal inverted microscope (e.g., Zeiss, LSM780) with a 63× oil immersion objective, 1.4 NA, and a femtosecond-pulsed infrared laser (Chameleon Compact OPO Fammily, Coherent).
Fiji version 1.53t, https://fiji.sc (Schindelin et al., 2012)
PIV analysis plugin version #2020/04/22, https://sites.google.com/site/qingzongtseng/piv (Tseng et al., 2012)
Equipment
Software
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sánchez-Cisneros, L. E., Bhide, S. and Ríos-Barrera, L. D. (2023). Recoil Measurements in Drosophila Embryos: from Mounting to Image Analysis. Bio-protocol 13(14): e4806. DOI: 10.21769/BioProtoc.4806.
- Ríos-Barrera, L. D. and Leptin, M. (2022). An endosome-associated actin network involved in directed apical plasma membrane growth. J. Cell Biol. 221(3): e202106124.
分类
生物物理学 > 显微技术 > 双光子激光扫描显微镜
细胞生物学 > 组织分析 > 组织成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link