- EN - English
- CN - 中文
An In-depth Guide to the Ultrastructural Expansion Microscopy (U-ExM) of Chlamydomonas reinhardtii
莱茵衣藻超微结构膨胀显微镜 (U-ExM) 的深入研究
发布: 2023年09月05日第13卷第17期 DOI: 10.21769/BioProtoc.4792 浏览次数: 4756
评审: Xin XuAnonymous reviewer(s)
Abstract
Expansion microscopy is an innovative method that enables super-resolution imaging of biological materials using a simple confocal microscope. The principle of this method relies on the physical isotropic expansion of a biological specimen cross-linked to a swellable polymer, stained with antibodies, and imaged. Since its first development, several improved versions of expansion microscopy and adaptations for different types of samples have been produced. Here, we show the application of ultrastructure expansion microscopy (U-ExM) to investigate the 3D organization of the green algae Chlamydomonas reinhardtii cellular ultrastructure, with a particular emphasis on the different types of sample fixation that can be used, as well as compatible staining procedures including membranes.
Graphical overview
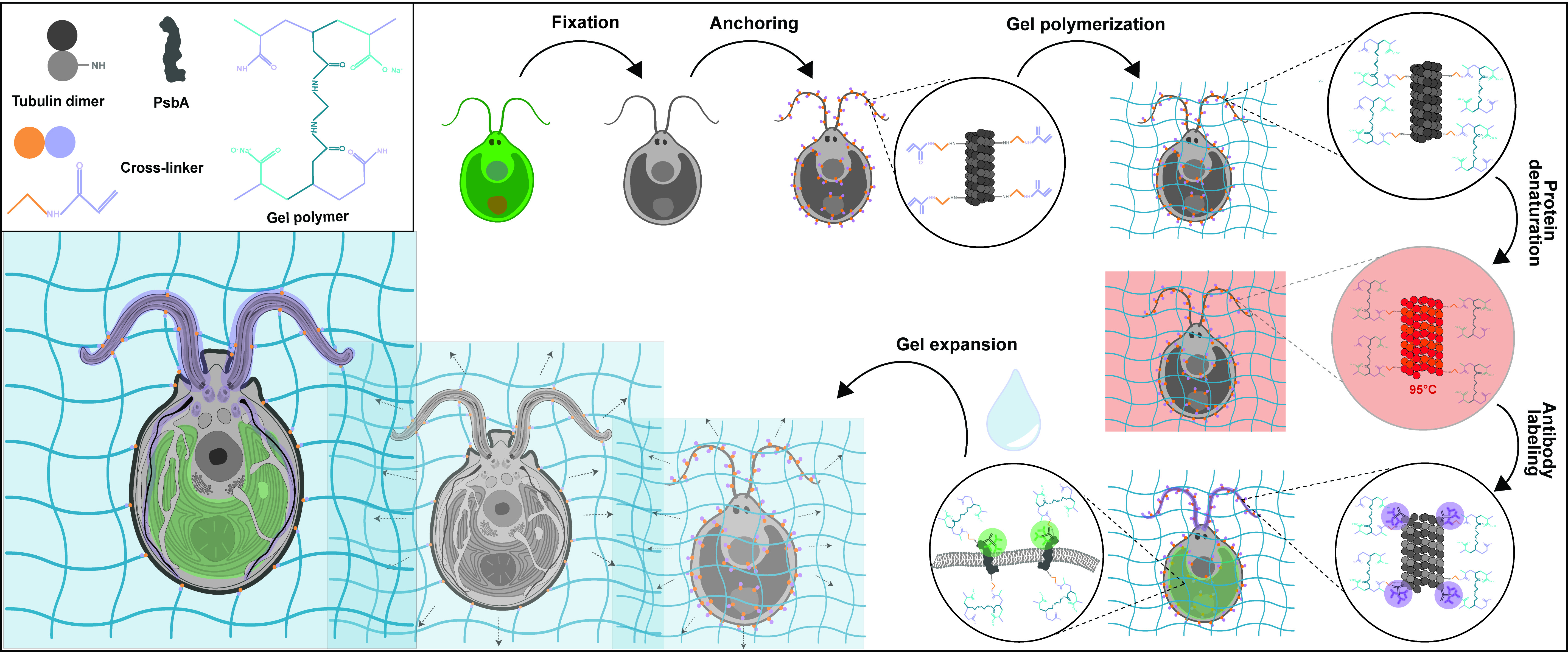
Background
Chlamydomonas reinhardtii, a single-celled green algae, is an ideal model organism in the fields of carbon fixation and ciliary-based motility, owing to its large chloroplast and two flagella. As such, electron microscopists have used Chlamydomonas for decades to unravel cellular questions about photosynthetic processes (Salomé and Merchant, 2019), basal body biogenesis and function (Dutcher, 2003), and flagellar motility (Klena and Pigino, 2022). However, the cellular localization of specific proteins of interest is difficult to address by electron microscopy (EM) and, when possible, it requires complex correlative light and EM approaches. Light microscopy and immunofluorescence microscopy are often used to visualize specific protein targets, but the resolution is often insufficient, and the cellular context is indeterminable. Furthermore, utilizing super-resolution techniques such as stochastic optical reconstruction microscopy and stimulated emission depletion requires highly specialized setups and complicated antibody labeling. To circumvent this, we recently utilized super-resolution ultrastructural expansion microscopy (U-ExM) on Chlamydomonas to assess how intraflagellar transport trains (IFT) assemble at the ciliary base (van den Hoek et al., 2022). U-ExM is an approach of expansion microscopy derived from the magnified analysis of the proteome protocol that allows for a physical, volumetric expansion of the entire proteome by a factor of 4 (Ku et al., 2016; Gambarotto et al., 2019). By coupling U-ExM with cryo-fixation (Laporte et al., 2022b), NHS-ester (a reactive dye interacting with amines)-mediated pan-ExM experiments (M’Saad and Bewersdorf, 2020), and BODIPYTM (conjugated dye to label lipids) membrane staining (Liffner and Absalon, 2021), the native ultrastructural environment of the entire organism can be assessed through light microscopy, and the localization of specific proteins of interest can be determined by immunofluorescence. The protocol presented here is dedicated to the expansion of Chlamydomonas cells but it can also be used for other organisms such as human cells (Laporte et al., 2022a), mouse tissue (Mercey et al., 2022), or yeast (Hinterndorfer et al., 2022).
Materials and reagents
Reagents
Chlamydomonas growth media
Tris(hydroxymethyl)aminomethane (TRIS) (Sigma, catalog number: 252859), store at room temperature (RT)
Hutner’s trace elements (Chlamydomonas Resource Center)
Potassium phosphate monobasic (KH2PO4) (Sigma, catalog number: P5655), store at RT
Potassium phosphate dibasic (K2HPO4) (Sigma, catalog number: P3786), store at RT
Acetic acid (Sigma, catalog number: A6283)
TRIS-acetate-phosphate (TAP) medium (see Recipes)
Phosphate buffer II (see Recipes)
Solution A (40×) (see Recipes)
Regular U-ExM protocol
Formaldehyde, 36.5%–38% (FA) (Sigma, catalog number: F8775), store at RT
Acrylamide, 40% (AA) (Sigma, catalog number: A4058), store at 4 °C
Sodium dodecyl sulfate (SDS) (Carl Roth, article number: CN30.3), store at RT
N,N’-methylenebisacrylamide, 2% (BIS) (Sigma, catalog number: M1533), store at 4 °C
Sodium acrylate, 97%–99% (SA) (Sigma, catalog number: 408220), store at -20 °C
Ammonium persulfate (APS) (Thermo Fisher, catalog number: 17874), store at RT
Tetramethylethylenediamine (TEMED) (Thermo Fisher, catalog number: 171919), store at RT
TWEEN 20 (Sigma, catalog number: P1379), store at RT
Bovine serum albumin (BSA) (Sigma, catalog number: A3059), store at 4 °C
Poly-D-lysine, 0.1 mg/mL (PDL) (Gibco, catalog number: A3890401), store at 4 °C
PBS (1× and 10×)
ddH2O
Nuclease-free water (Thermo Fisher Scientific, catalog number: AM9937)
Antibodies (primaries and secondaries)
NHS-ester Atto488 (Sigma, catalog number: BCCJ6663)
NHS-ester 405 (Sigma, catalog number: 250966)
NHS-ester Atto 594 (Sigma, catalog number: 08AA12)
NHS-ester Atto 647 (Sigma, catalog number: BCCD9924)
BODIPY 558/568 (Red) (Thermo Fisher Scientific, catalog number: D3835)
Paraformaldehyde (PFA), 16% (EMS, catalog number: 15710)
Glutaraldehyde (GA), 25% (Sigma, catalog number: G5882)
Methanol (MeOH) (Sigma, catalog number: 34860)
α-tubulin antibody (ABCD Antibodies, catalog number: AA345)
β-tubulin antibody (ABCD Antibodies, catalog number: AA344)
SNAP-tag antibody (New England Biolabs, catalog number: P9310S)
PsbA antibody (Agrisera, AS06 143)
Centrin antibody clone 20H5 (Millipore, catalog number: 04-1624)
Formaldehyde/acrylamide mixture (1.4% FA, 2% AA) (see Recipes)
Sodium acrylate solution (38% w/w) (see Recipes)
Monomer solution, 10 aliquots of 90 μL (see Recipes)
10% TEMED, 10 aliquots of 100 μL (see Recipes)
10% APS, 10 aliquots of 100 μL (see Recipes)
Denaturation buffer, pH 9, 100 mL (see Recipes)
PBS-Tween (0.1% w/v) (1×, 1 L) (see Recipes)
PBS-BSA (2%) (1×, 100 mL) (see Recipes)
Additional reagents for Cryo-ExM protocol
Acetone (99.8% AcroSeal) (Acros Organics, catalog number: 67-64-1)
Ethanol (EtOH, absolute) (Thermo Fisher Scientific, catalog number: 397691000)
Dry ice
Ethane gas bottle (PanGas). It is also possible to use a 37%:63% mixture of ethane:propane (PanGas), which avoids the freezing of the gas during the preparation
Materials
Regular U-ExM protocol
Erlenmeyer flasks
Tweezer Dumont Style 5 with super thin tips (0203-L5-PO) or Negative-Action tweezer Style no. 5 (0203-N5-PO)
12 mm coverslips, high precision no. 1.5H (Marienfeld Laboratory Glassware, catalog number: 47442)
24 mm coverslips, high precision no. 1.5H (Marienfeld Laboratory Glassware, catalog number: 48639)
12-well plates (Thermo Fisher Scientific, catalog number: 150200)
6-well plates (Thermo Fisher Scientific, catalog number: 150239)
P1000 pipette tips
P200 pipette tips
P20 pipette tips
Spatula (Bochem, catalog number: 3101)
Spoon (Bochem, catalog number: 3421)
Razor blade (Carl-Roth, catalog number: CK07.2)
250 mL beaker (VWR, catalog number: 213-1124)
500 mL beaker (VWR, catalog number: 213-1126)
Caliper
Fine-mesh net (such as mosquito netting or insect netting for a garden)
Parafilm (BemisTM, catalog number: PM996)
Scissors
Pasteur pipettes (Alpha Laboratories, catalog number: LW4111)
1.5 mL Eppendorf tubes
35 mm imaging chamber (metallic O-ring 35 mm) (Okolab, catalog number: RA-35-18 2000–06)
Coverslip rack (Thermo Fisher Scientific, catalog number: C14784)
Additional material for Cryo-ExM protocol
Polystyrene box (23 cm × 23 cm × 21 cm)
5 mL Eppendorf tubes (Eppendorf, catalog number: 0030119401)
Tweezer Dumont Style L5 Inox 8 with clamping ring (Electron Microscopy Sciences)
Whatman filter paper, 55 mm (GE Healthcare, catalog number: 1001-055)
Equipment
Regular U-ExM protocol
37 °C incubator or climate-controlled room
Heat block (Cole-ParmerTM, catalog number: SBH130DC)
Inverted fluorescent microscope, such as widefield Leica Thunder, SP8, or Zeiss LSM 980, equipped with a 63× oil objective.
Additional equipment for Cryo-ExM protocol
Cryo-EM manual plunger [both devices used in the preparation of this manuscript were homemade plunging devices; however, commercial manual cryo-plunging devices are usable (https://www.mitegen.com/product/manual-plunge-cooler/), and homemade manual plunging devices can be produced by groups (Comolli et al., 2012)].
Software
Fiji (ImageJ, https://imagej.net/software/fiji/) (Schindelin et al., 2012)
Depending on the brand of microscope used for imaging, dedicated software is required, such as:
LAS X (Leica, https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/)
Zen Blue (Zeiss, https://www.zeiss.com/microscopy/en/products/software/zeiss-zen.html)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Klena, N. T., Maltinti, G., Batman, U., Pigino, G., Guichard, P. and Hamel, V. (2023). An In-depth Guide to the Ultrastructural Expansion Microscopy (U-ExM) of Chlamydomonas reinhardtii. Bio-protocol 13(17): e4792. DOI: 10.21769/BioProtoc.4792.
- van den Hoek, H., Klena, N., Jordan, M. A., Alvarez Viar, G., Righetto, R. D., Schaffer, M., Erdmann, P. S., Wan, W., Geimer, S., Plitzko, J. M., et al. (2022). In situ architecture of the ciliary base reveals the stepwise assembly of intraflagellar transport trains. Science 377(6605): 543–548.
分类
植物科学 > 植物细胞生物学 > 细胞成像
细胞生物学 > 细胞成像 > 固定细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












