- EN - English
- CN - 中文
VAR2CSA Ectodomain Labeling in Plasmodium falciparum Infected Red Blood Cells and Analysis via Flow Cytometry
恶性疟原虫感染红细胞的VAR2CSA外结构域标记及其流式细胞术分析
发布: 2023年08月05日第13卷第15期 DOI: 10.21769/BioProtoc.4725 浏览次数: 1309
评审: Kathrin SutterAnonymous reviewer(s)
Abstract
Presentation of the variant antigen Plasmodium falciparum erythrocyte membrane protein 1 (EMP1) at the surface of infected red blood cells (RBCs) underpins the malaria parasite’s pathogenicity. The transport of EMP1 to the RBC surface is facilitated by a parasite-derived trafficking system, in which over 500 parasite proteins are exported into the host cell cytoplasm. To understand how genetic ablation of selected exported proteins affects EMP1 transport, several EMP1 surface presentation assays have been developed, including: 1) trypsinization of surface-exposed EMP1 and analysis by SDS-PAGE and immunoblotting; and 2) infected RBC binding assays, to determine binding efficiency to immobilized ligand under physiological flow conditions. Here, we describe a third EMP1 surface presentation assay, where antibodies to the ectodomain of EMP1 and flow cytometry are used to quantify surface-exposed EMP1 in live cells. The advantages of this assay include higher throughput capacity and data better suited for robust quantitative analysis. This protocol can also be applied to other cellular contexts where an antibody can be developed for the ectodomain of the protein of interest.
Keywords: Malaria (疟疾)Background
The most virulent form of malaria is caused by the protozoan parasite Plasmodium falciparum, killing over 600,000 people annually (Weiss et al., 2019). During the asexual blood stage, the parasite invades the red blood cell (RBC) and feeds on intracellular hemoglobin; however, while circulating in the host bloodstream, the infected RBC is at risk of elimination if it transits the host’s splenic sinuses. To avoid splenic clearance, the parasite remodels the host cell by exporting proteins into the RBC cytoplasm (Marti et al., 2004; Sargeant et al., 2006; Boddey et al., 2013; Heiber et al., 2013). During this process, a key modification is the presentation of the variant antigen P. falciparum erythrocyte membrane protein 1 (EMP1) at the surface of infected RBCs (Smith et al., 1995). The antigen EMP1 is encoded by the var gene family where each parasite expressing one of approximately 60 variants at any time. These variants act as adhesins to a range of cellular ligands, expressed on a range of endothelial cells in the capillaries throughout the body, allowing the infected RBC to sequester within the host’s vasculature. The cytoadhesion of infected RBCs can lead to fatal complications associated with cerebral and placental malaria (Storm and Craig, 2014; Jensen et al., 2020; Sahu et al., 2021). EMP1 transport to the surface remains poorly understood as the parasite, with the parasite building its own de novo trafficking system, which is highly divergent from classical eukaryotic trafficking machinery. For these reasons, EMP1 trafficking is of high interest from both clinical and basic biology perspectives.
In our recent studies, we identified parasite proteins that are exported into the host cell and affect the trafficking and presentation of the antigen EMP1 (McHugh et al., 2020; Carmo et al., 2022). The majority of EMP1 is trafficked to the host cell surface 16–20 h post invasion, and mid-trophozoite stage-infected RBCs (20–32 h post invasion) are used to study EMP1 presentation at the host cell surface (Kriek et al., 2003). To determine if EMP1 is present at the surface of infected RBCs, we employ two complementary assays. These include: 1) trypsin cleavage assay, where surface-exposed EMP1 is shaved from the surface by trypsin and the membrane-embedded domain is then detected by immunoblotting (Cooke et al., 2006; Carmo et al., 2022); and 2) binding assays, in which the infected RBCs are passed through a channel coated with ligand and the number of cells adhering is quantified (McHugh et al., 2015 and 2020; Carmo et al., 2022). These assays are useful; however, they have their limitations. For example, the trypsin assay is semi-quantitative at best, while defects in adhesion underflow can be multifactorial (not due to EMP1 surface presentation alone).
In an alternative approach outlined here, antibodies specific to the ectodomain of EMP1 are used to label live cells, which are then quantitated by flow cytometry, to measure the number of labeled cells and their relative intensity of surface-exposed EMP1 (first established in Smith et al., 1995; Beeson et al., 2004; Elliott et al., 2005) (Figure 1). Of the techniques assaying EMP1 surface presentation published to date, this is the most time- and resource-efficient method. In addition, the assay is implemented in a 96-well plate format, making it easily scalable. This technique can also be adjusted to quantitate surface presentation of surface proteins in other cellular contexts where an antibody is available for the ectodomain of the protein of interest.
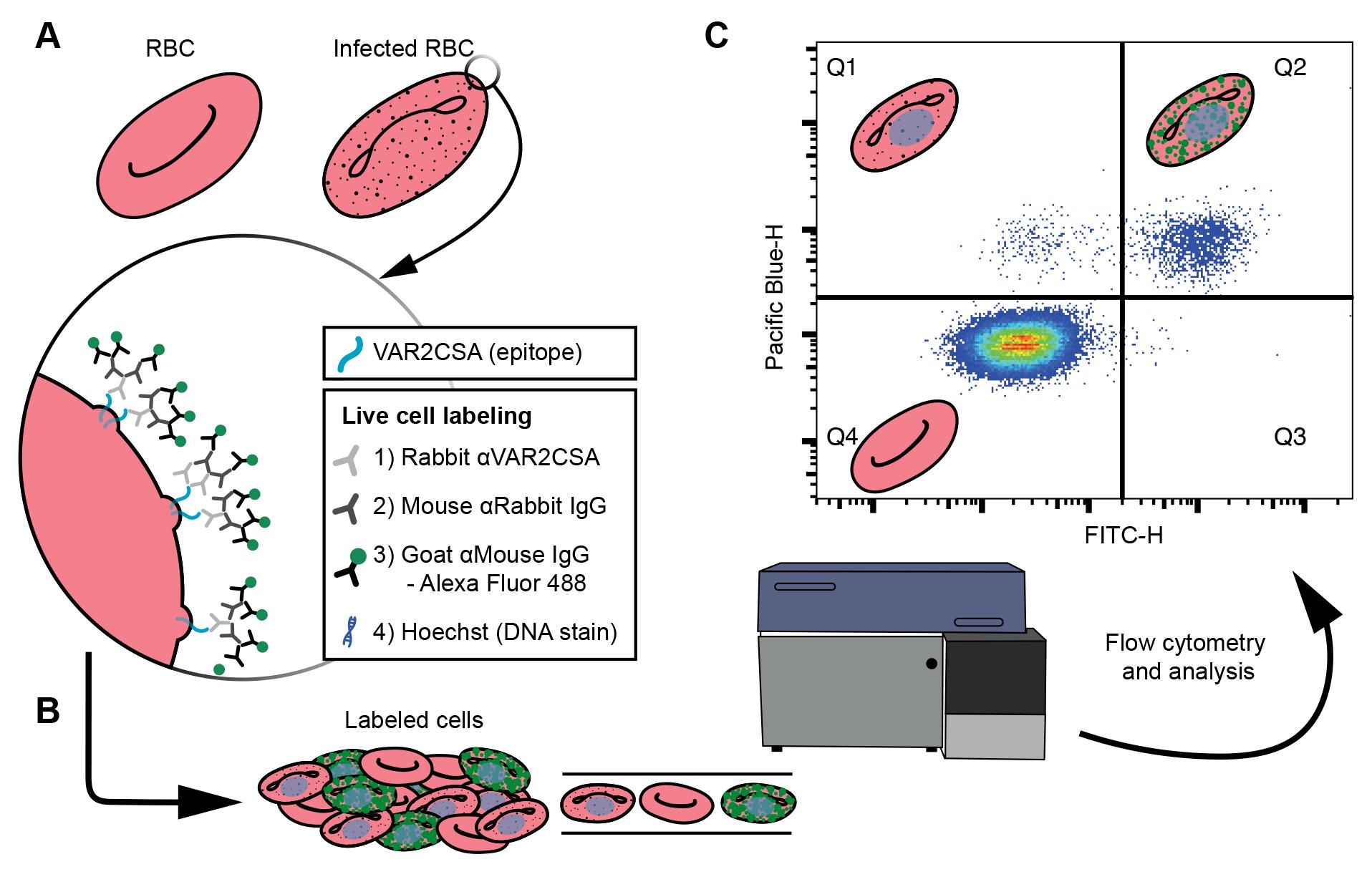
Figure 1. Cell labeling and analysis flowchart. (A) Live cell labeling of the ectodomain of the VAR2CSA, an EMP1 variant available on the surface of P. falciparum infected red blood cells (RBCs). The VAR2CSA variant is associated with adhesion to chondroitin sulphate A in the placenta, causing placental malaria. A zoom-in window depicts the VAR2CSA antigen (blue) with a list of the live cell labeling steps: incubations with three antisera, the final of which is conjugated to an Alexa Fluor 488, and finally a DNA stain. (B) The labeled cells are analyzed by flow cytometry. (C) Major populations can be separated by plotting the Pacific blue height (cells with DNA) against FITC height (cells labeled with Alexa Fluor 488), where each dot is a single cell and high densities of cells are indicated by blue through to red hues. Schematics of RBCs indicate the type of cell gated in each quartile. Uninfected RBCs do not have DNA; therefore, Q4 represents uninfected cells. Q1 represents infected cells that do not have VAR2CSA ectodomains available to the antisera. Q2 indicates infected cells that have VAR2CSA ectodomains available to the antisera. These data are used to compare the Q2 populations between cell lines to determine if mutations to P. falciparum cell lines affect VAR2CSA translocation to the surface of the infected RBC.
Materials and reagents
Storage notes: All primary antibodies are stored at -20 °C. Secondary antibodies and DNA stains are stored at 4 °C unless otherwise noted. All reagents for tissue culture were prepared in a class II biosafety cabinet while practicing aseptic technique, including the use of sterile pipette tips.
96-well plate, v-well, polystyrene with lid, sterile (Sarstedt, catalog number: 3 82.1583.001)
Sterile 35 mm × 10 mm tissue culture dish (Sigma, Corning, catalog number: 430165)
BSA 30% (w/v) (CSL Immulab, catalog number: 06701305)
Rabbit anti-VAR2CSA antibody recognizing an epitope on the ectodomain of VAR2CSA [Duffy and Rogerson labs, University of Melbourne, code: R1945 (Reeder et al., 1999)]
Monoclonal anti-rabbit IgG (γ-chain specific) antibody produced in mouse (Sigma, catalog number: R1008-2ML)
Alexa Fluor 488 goat anti-mouse IgG (H+L) (Invitrogen, Life Technologies, catalog number: A11029)
Alexa Fluor 647 goat anti-mouse IgG (H+L) (Invitrogen, Life Technologies, catalog number: A21236)
Hoechst 33342 (Invitrogen, catalog number: H3570)
SYTO 61 (Life Technologies, catalog number: S11343) to measure parasitemia. SYTO 61 is diluted from 5 mM to 100 μM in DMSO and stored in 50 μL aliquots at -20 °C
Dimethyl sulfoxide (DMSO) (ChemSupply, catalog number: DA013-500M)
RPMI 1640 medium with GlutaMAX supplement and HEPES (Life Technologies, Gibco, catalog number: 72400120)
Hypoxanthine (Sigma, catalog number: H9636-5G) made to 200 mM in 1 M NaOH (ChemSupply, catalog number: SL178-500G), filter sterilized, and stored in 1 mL aliquots at -20 °C
Gentamicin 10 mg/mL in deionized water (Sigma, catalog number: G1397)
D-glucose (ChemSupply, catalog number: GA018-500G)
D-sorbitol (ChemSupply, catalog number: SL151-500G), made to 5% w/v in MilliQ water, filter sterilized, and stored at 4 °C
Albumax II (Life Technologies, catalog number: 11021045) dissolved in RPMI 1640 medium with GlutaMAX supplement and HEPES at 5%, filter sterilized, and stored in 25 mL aliquots at -20 °C
Media solution (910 mM D-glucose, 0.45 mg/mL gentamicin), stored in 5 mL aliquots at -20 °C
Pooled sera from any blood type (Lifeblood Australia), heat inactivated for 1.5 h, filter sterilized, and stored in 25 mL aliquots at -20 °C
Red blood cells (Lifeblood Australia), O+
Malaria mix (1% O2, 5% CO2, and 94% N2) (Coregas, catalog number: 388150)
Giemsa’s stain improved solution R66, Gurr for microscopical staining (VWR chemicals, catalog number: 350864X)
Methanol (ChemSupply, catalog number: MA004)
Triton X-100 (BioXtra, Sigma, catalog number: T9284)
Sodium hypochlorite 8-14% (Ajax Finechem, catalog number: AJA485-5L)
Complete culture media (CCM) stored at 4 °C, used at 37 °C (see Recipes)
Equipment
Incubator (LabQuip Sciences, SEM Equipment, model: 18FD)
Multichannel pipettes, 8-channel, 20–200 μL (Socorex, model: 855) and 5–100 µL (Bohit, model: m100)
Class II biosafety cabinet (Laftech, EuroClone, model: safemate 1.2 vision)
Centrifuge (Hettich, model: Rotina 420)
Flow cytometer [BD Biosciences, model: FACSCanto II Flow Cytometer System with an integrated BD High Throughput Sampler (HTS)]. The following filter sets were used for fluorophore detection: Alexa Fluor 647 and SYTO-61 (APC, 660/20 nm), Alexa Fluor 488 (FITC, 530/30 nm), and Hoechst 33342 (Pacific Blue, 450/50 nm)
Software
FACSDiva 8.0.1 (BD Biosciences, https://www.bdbiosciences.com/en-au/products/software/instrument-software/bd-facsdiva-software)
FlowJo v10 (BD Biosciences, https://www.flowjo.com/solutions/flowjo)
Prism 9 (Dotmatics, GraphPad, https://www.graphpad.com/scientific-software/prism/)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Carmo, O. M. S. and Dixon, M. W. A. (2023). VAR2CSA Ectodomain Labeling in Plasmodium falciparum Infected Red Blood Cells and Analysis via Flow Cytometry. Bio-protocol 13(15): e4725. DOI: 10.21769/BioProtoc.4725.
分类
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
免疫学 > 宿主防御 > 人
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link