- EN - English
- CN - 中文
Monitoring Group 2 Innate Lymphoid Cell Biology in Models of Lung Inflammation
监测组2肺部炎症模型中的先天淋巴细胞生物学
发布: 2023年07月20日第13卷第14期 DOI: 10.21769/BioProtoc.4717 浏览次数: 2681
评审: Meenal SinhaJulie WeidnerLuis Alberto Sánchez Vargas
Abstract
Innate lymphoid cells (ILCs) are a rare cell population subdivided into ILC1s, ILC2s, and ILC3s, based on transcription factor expression and cytokine production. In models of lung inflammation, the release of alarmins from the epithelium activates ILC2s and promotes the production of Th2-cytokines and the proliferation and migration of ILC2s within the lung. ILC2s are the innate counterpart to CD4+ Th2s and, as such, express Gata-3 and produce IL-4, IL-5, and IL-13. Due to the low number of ILCs and the lack of specific surface markers, flow cytometry is the most reliable technique for the identification and characterization of ILCs. In this protocol, multicolor flow cytometry is utilized to identify Lineage−Thy1.2+ ILCs. Intracellular cytokine staining further identifies ILC2s within the lung. This protocol presents a reliable method for promoting ILC2-mediated lung inflammation and for monitoring ILC2 biology.
Key features
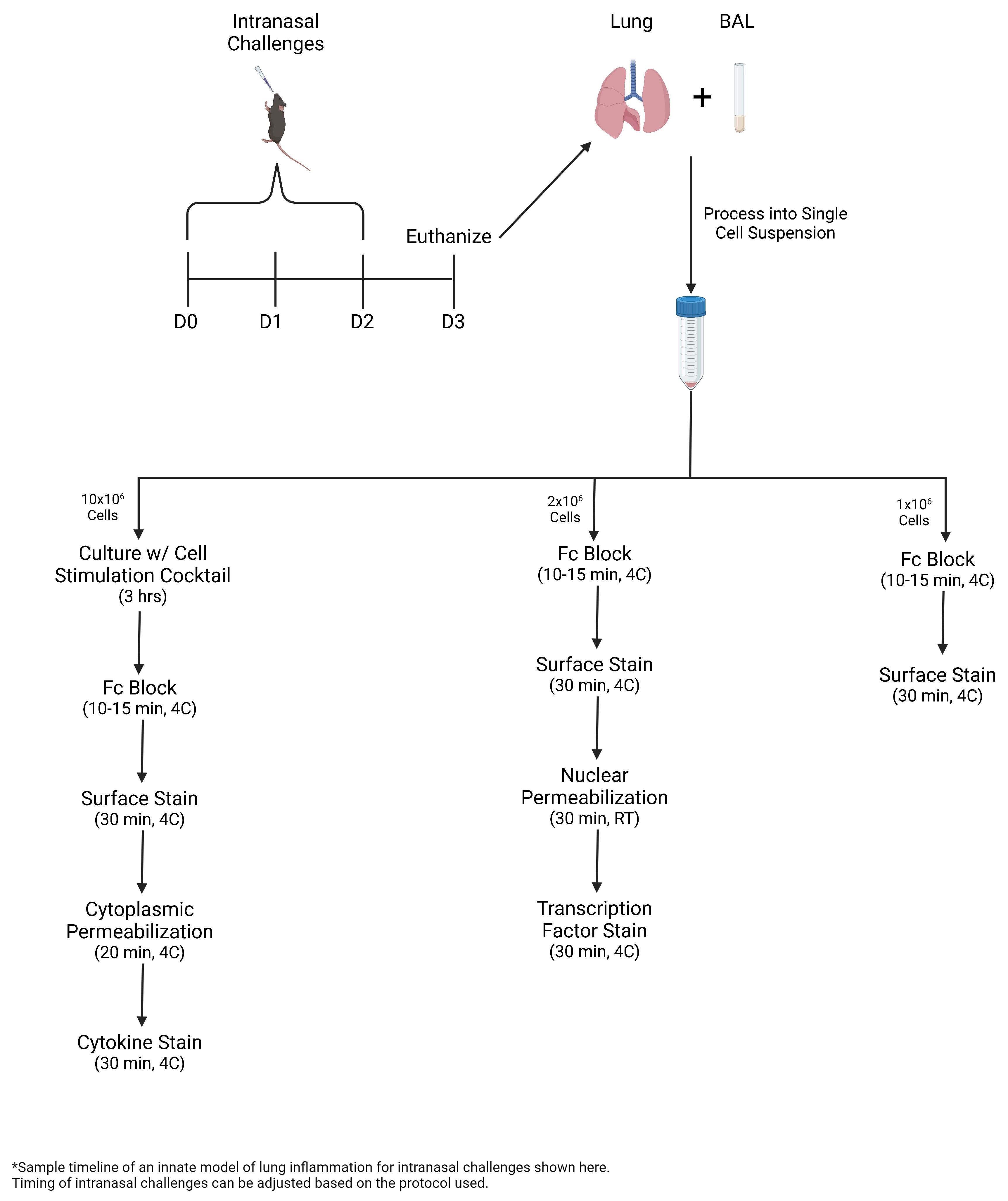
• In this protocol, ILC2s are expanded via intranasal challenges with Alternaria alternata, a fungal allergen, or recombinant IL-33.
• Bronchoalveolar lavage (BAL) and lung are collected and processed into single-cell suspension for multicolor flow cytometric analysis, including intracellular staining of transcription factors and cytokines.
• During lung inflammation, the percentage of ILC2s and eosinophils increases. ILC2s express greater levels of Gata-3 and Ki-67 and produce greater amounts of IL-5 and IL-13.
Graphical overview
Background
Innate lymphoid cells (ILCs) are a heterogeneous population of lymphocytes that lack common lineage markers. ILCs are differentiated by their transcription factor expression profiles and cytokine production capabilities (Wirtz et al., 2021). ILCs are categorized into three major subpopulations, ILC1s, ILC2s, and ILC3s, which are the innate counterparts to CD4+ Th1, Th2, and Th17 cells (Artis and Spits, 2015). ILCs and their T-cell counterparts share similar functions, transcription factor profiles, and cytokine production. ILC1s and Th1s express T-bet and produce IFNγ in response to intracellular pathogens. ILC2s and Th2s express Gata-3 and produce IL-4, IL-5, and IL-13 in response to extracellular pathogens and allergens. ILC3s and Th17s express RORγt and produce IL-17a in response to extracellular pathogens (Drake et al., 2014; Vivier et al., 2018).
In the lung, ILC2s are the most common ILC subpopulation (Gasteiger et al., 2015). Lung-resident ILC2s can be identified by their expression of Thy-1, CD127 (IL-7 receptor), CD25 (IL-2 receptor), and T1ST2 (IL-33 receptor) (Monticelli et al., 2011). Lung inflammation is associated with the epithelial release of alarmins, including IL-33, IL-25, and thymic stromal lymphopoietin (Rossi et al., 2022). ILC2s respond to alarmins and mediate inflammation within the lungs; in response to environmental allergens, ILC2s produce their effector cytokines and expand locally (Lai et al., 2016). ILC2-derived IL-4 is important for the differentiation of naïve T cells into Th2 cells and the transition to adaptive immunity (Pelly et al., 2016). IL-5 plays a role in maintaining and promoting eosinophils (Ikutani et al., 2017), while IL-13 promotes mucus secretion (Varela et al., 2022). With inflammation, lung ILC2s are more motile and accumulate in peribronchial and perivascular spaces (Puttur et al., 2019).
In addition to being a rare cell population, ILCs lack specific and unique surface markers, which presents a challenge in identifying ILCs in tissues. Flow cytometry is the most established technique for identifying and characterizing ILCs, including the different subpopulations. Multicolor flow cytometry allows for the identification of multiple populations based on multiple parameters. For ILC populations, a combination of surface markers, transcription factors, and cytokines serves as the basis for identifying these populations. Here, murine ILCs are identified as Lineage−Thy1.2+ lymphocytes. IL-5 and IL-13 production is used to further identify pulmonary ILC2s. In this protocol, ILC2s are expanded via intranasal challenges with Alternaria alternata, a fungal allergen, or recombinant IL-33. Bronchoalveolar lavage and lung are collected and processed into single-cell suspension for multicolor flow cytometric analysis, including intracellular staining of transcription factors and cytokines.
Materials and reagents
21 G needle (BD, catalog number: 305167)
18 G needle (BD, catalog number: 305196)
1.5 mL Eppendorf tubes (Genesee Scientific, catalog number: 24-282S)
Flat-bottom 96-well plate (Genesee Scientific, catalog number: 25-109)
70 μm filter (Corning, catalog number: 431751)
15 mL conical tubes (FroggaBio, catalog number: TB15-25)
50 mL conical tubes (Falcon, catalog number: 352098)
Suture (Look, catalog number: SP105)
gentleMACS c-tube (Miltenyi Biotec, catalog number: 130-093-237)
Polystyrene FACS tubes (Fisher Scientific, catalog number: 14-959-5)
C57BL/6 mice (6–8 weeks old; female)
Miltenyi Lung Digest kit (Miltenyi Biotec, catalog number: 130-095-927)
Mouse recombinant IL-33 (Thermo Fisher, catalog number: 14-8332-80)
Alternaria alternata extract (Fisher Scientific, catalog number: NC1620293)
1× PBS (Gibco, catalog number: 10010-023)
RPMI (Gibco, catalog number: 21870-076)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906-100G)
Fetal bovine serum (FBS) (Corning, catalog number: 35-015-CV)
Sodium azide (Fisher Scientific, catalog number: BP922I-500)
Penicillin/streptavidin (Gibco, catalog number: 15140122)
L-Glutamine (Gibco, catalog number: 25030-081)
β-Mercaptoethanol (Gibco, catalog number: 21985-023)
Cell stimulation cocktail plus protein transport inhibitor (Thermo Fisher Scientific, catalog number: 00-4975-93)
PE anti-mouse IL-13 (Thermo Fisher, catalog number: 12-7133-82; clone: eBio13A, dilution: 1:20)
PE anti-mouse/human IL-5 (BioLegend, catalog number: 504304; clone: TRFK5, dilution: 1:20)
PE Ki-67 monoclonal antibody (Thermo Fisher, catalog number: 12-5698-82; clone: SolA15, dilution: 1:50)
APC anti-mouse Ly6G-Ly6C (GR1) (BioLegend, catalog number: 108412; clone: RB6-8C5, dilution: 1:50)
PE anti-mouse SiglecF (BD Biosciences, catalog number: 552126; clone: E502440, dilution: 1:50)
PerCP anti-mouse CD45.2 (BioLegend, catalog number: 109828; clone: 104, dilution: 1:50)
FITC anti-mouse TCR γ/δ (BioLegend, catalog number: 118106; clone: GL3, dilution: 1:200)
FITC anti-mouse TCR β Chain (BioLegend, catalog number: 109206; clone: H57-597, dilution: 1:200)
FITC anti-mouse FceR1α (BioLegend, catalog number: 134306; clone: MAR-1, dilution: 1:200)
FITC anti-mouse CD5 (BioLegend, catalog number: 100606; clone: 53-7.3, dilution: 1:200)
FITC anti-mouse NK-1.1 (BioLegend, catalog number: 108706; clone: PK136, dilution: 1:200)
FITC anti-mouse CD11c (BioLegend, catalog number: 117306; clone: N418, dilution: 1:200)
FITC anti-mouse Lineage (BioLegend, catalog number: 133302; clone: 145-2C11; RB6-8C5; RA3-6B2; Ter-119; M1/70, dilution: 1:40)
Purified anti-mouse CD16/32 (Fc Block) (BioLegend, catalog number:101302; clone: 93, dilution: 1:50)
BD Cytofix/Cytoperm Fixation/Permeabilization kit (Fisher Scientific, catalog number: BDB554714)
FoxP3/Transcription Factor Staining Buffer Set (FoxP3 Perm kit and buffer) (ThermoFisher, catalog number: 00-5523-00)
32% paraformaldehyde (PFA), EM grade (Electron Microscopy Sciences, catalog number: 15714) (diluted to 4% with DI water)
FACS buffer (see Recipes)
T-cell media (see Recipes)
Equipment
Single-channel pipettes (Rainin: P10, P200, P1000)
Multi-channel pipette (Thermo Scientific)
Catheter tubing (BD, catalog number: 427411)
Fine forceps (Fine Science Tools, catalog number: 11412-11)
Curved forceps (Fine Science Tools, catalog number: 11054-10)
Surgical scissors (Fine Science Tools, catalog number: 14060-10)
Centrifuge (Beckman Coulter, model: Allegra X-15R, swinging bucket rotor)
gentleMACS dissociator, octo dissociator (Miltenyi Biotec, catalog number: 130-096-427)
gentleMACS mixer, octo dissociator (Miltenyi Biotec, catalog number: 130-096-427)
Incubator (Panasonic, catalog number: MCO-18ACL-PA)
Novocyte 3000 (Acea, catalog number: 2010011)
Software
FlowJo (Version 10.8.0)
NovoExpress (Agilent)
Prism (GraphPad, Version 9.2.0)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Badrani, J. H., Strohm, A. N., Huang, Y. A. and Doherty, T. A. (2023). Monitoring Group 2 Innate Lymphoid Cell Biology in Models of Lung Inflammation. Bio-protocol 13(14): e4717. DOI: 10.21769/BioProtoc.4717.
分类
免疫学 > 动物模型 > 小鼠
免疫学 > 免疫细胞染色 > 免疫检测
细胞生物学 > 细胞染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link