- EN - English
- CN - 中文
Iterative Indirect Immunofluorescence Imaging (4i) on Adherent Cells and Tissue Sections
粘附细胞和组织切片的迭代间接免疫荧光成像(4i)
(*contributed equally to this work) 发布: 2023年07月05日第13卷第13期 DOI: 10.21769/BioProtoc.4712 浏览次数: 3743
评审: Chiara AmbrogioAnthony FlamierAkira Karasawa

相关实验方案
自动层次分析(ALAn):用于无偏见表征培养中哺乳动物上皮结构的图像分析工具
Christian Cammarota [...] Tara M. Finegan
2024年04月20日 4217 阅读
Abstract
Highly multiplexed protein measurements from multiple spatial scales using fluorescence microscopy recently emerged as a powerful way to investigate tumor microenvironments in biomedicine and the multivariate nature of complex systems’ interactions. A range of methods for this exist, which either rely on directly labeling the primary antibody with oligonucleotides/rare metals or employing methods to remove fluorescence for cyclic acquisition. Here, we describe a protocol that uses off-the-shelf primary and secondary antibodies without further need for modification and only commonly available chemical reagents. The method harnesses the observation that antibodies can crosslink to bound epitopes during light exposure, thus preventing elution. By utilizing a simple oxygen radical scavenging buffer during imaging and by blocking free sulfhydryl groups before antibody incubation, the presented method can employ comparably mild conditions to remove bound antibodies from epitopes, which preserves sample integrity. Thus, with the stated minor modifications, it allows for a standard immunofluorescence imaging protocol in cyclic fashion, currently permitting staining of up to ~80 unique epitopes.
Graphical overview
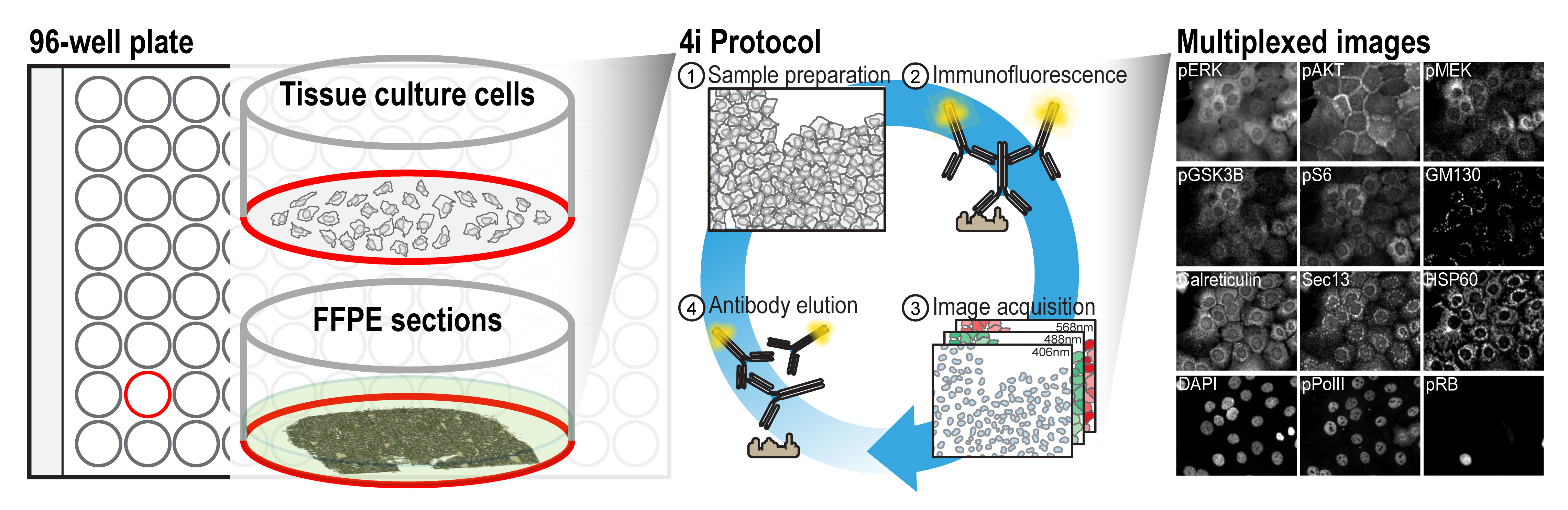
Background
A multitude of research questions across various fields of biology can only be addressed by the ability to simultaneously visualize the abundance and subcellular distribution of proteins in single cells, within their tissue context. Multiplexed imaging enables the identification of multiple cell types in a single sample, which facilitates the characterization of tissue microenvironments and cellular ecosystems in biomedicine (Cole et al., 2022; Wahle et al., 2022). It further enables an in-depth description of the multivariate nature of cellular states, which are predictive of higher-level biological phenomena in basic research. Given the substantial research potential of multiplexed imaging methods, multiple methods have recently been developed, as outlined in Hickey et al. (2022). These methods rely either on directly conjugating the primary antibodies to oligonucleotides or metal-chelating moieties that can bind rare metals, which requires additional processing of the antibodies, or employ chemical conditions to elute the bound antibodies, which might affect sample integrity. For researchers who wish to use multiplexed imaging but are not specialists in these methods, or who require the detection of epitopes for which no modified antibodies exist, a protocol that uses off-the-shelf antibodies would be highly beneficial.
During fluorescence imaging, the generated oxygen radicals can crosslink antibodies to their bound epitopes, thereby hindering antibody removal. By employing a radical scavenging buffer during imaging, this crosslinking can be prevented, allowing antibody removal using relatively mild conditions. This allows for cyclic imaging using a conventional immunofluorescence staining protocol and off-the-shelf primary and secondary antibodies.
The ease of application of the described protocol, lack of need for specialized equipment, and the reliance on off-the-shelf antibodies and commonly available reagents allows researchers with various backgrounds to easily obtain multiplexed images of their sample of interest. While here we describe the protocol for a conventional 96-well imaging plate, using adherent cell lines and formalin-fixed paraffin-embedded (FFPE) sections, the procedure can readily be extended to a variety of samples and formats. For instance, it can readily be employed on coverslips, 384-well plates, and various other formats with minor adaptations. Combined with automated liquid-handling equipment, the approach can be scaled up.
Materials and reagents
For both tissue culture cells and FFPE tissue sections
L-Glycine-hydrochloride (Sigma-Aldrich, catalog number: G2879); storage: room temperature (RT)
Guanidium-hydrochloride (Sigma-Aldrich, catalog number: G3272); storage: RT
Urea (Sigma-Aldrich, catalog number: U5128); storage: RT
Tris(2-carboxyethyl)phosphine hydrochloride (Sigma-Aldrich, catalog number: C4706); storage: 4 °C
N-acetyl-cysteine (Sigma-Aldrich, catalog number: A7250); storage: 4 °C
Bovine serum albumin (Sigma-Aldrich, catalog number: A9418); storage: 4 °C
Maleimide (Sigma-Aldrich, catalog number: 129585); storage: 4 °C
Phosphate-buffered saline (PBS) (Thermo Fisher, catalog number: 10010023); storage: RT
1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Thermo Fisher, catalog number: 15630080); storage: 4 °C
12 M hydrochloric acid (Sigma-Aldrich, catalog number: 320331); storage: RT
10 M sodium hydroxide (Sigma-Aldrich, catalog number: 72068); storage: RT
Primary antibodies (various manufacturers); storage: according to manufacturers’ recommendations
Secondary antibodies (Thermo Fisher, choose according to color requirements and species of primary antibodies. We recommend Donkey-anti-Primary antibodies); storage: according to manufacturers’ recommendations
4’,6-Diamidino-2-phenylindole (Thermo Fisher, catalog number: 62247); storage: -20 °C
Specific for tissue culture cells
96-well imaging plate (Greiner, catalog number: 655096); storage: RT
16% paraformaldehyde (Electron Microscopy Sciences, catalog number: 15710); storage: RT
Triton-X (Sigma-Aldrich, catalog number: X100); storage: RT
Succinimidyl Ester Alexa Fluor 647 (Thermo Fisher, catalog number: A20006)
Specific for FFPE tissue sections
Glass bottom Nexterion (Schott Glass, catalog number: 1535661)
Grace Bio-Labs ProPlate MPTM microtiter plate superstructure (Sigma-Aldrich, catalog number: GBL204969-1EA)
National DiagnosticsTM Histo-ClearTM tissue clearing agent (Chemie Brunschwig AG, catalog number: D1620333)
100% EtOH (chemical under K20 chemical hood, was diluted in ddH2O)
Feather A35 1 × 50 Blades (Biosystems Switzerland AG, catalog number: 81-0358-00); storage: RT
30% w/v acrylamide/0.8% w/v bis-acrylamide (Sigma-Aldrich, catalog number: A3699-100ML); storage: 4 °C
TEMED (Thermo Fisher, catalog number: 17919); storage: 4 °C
Poly-L-lysine solution (Sigma-Aldrich, catalog number: P4832-50ML); storage: 4 °C
Glass basin length 21 cm × 15 cm × 6 cm
Superfrost plus microscope slides, 1 × 72 slides, 25 mm × 75 mm × 1 mm (Menzer-Glaseritem, catalog number: J1800AMNZ); storage: RT
PAP pen (Electron Microscopy Science, catalog number: 71310); storage: RT
Water-resistant, ethanol-soluble pen, 1 × 72 (Staedtler Lumocolor® permanent pen 318)
Histoclear clearing agent (National Diagnostic, catalog number: HS-200)
Solutions
40 mL elution buffer (EB) (see Recipes)
6 mL 4i blocking buffer (sBB) (see Recipes)
10 mL conventional blocking buffer (cBB) (see Recipes)
20 mL 4i imaging buffer (IB) (see Recipes)
20 mL fixation solution (FS) (see Recipes)
10 mL permeabilization solution (PS) (see Recipes)
5 mL primary antibody staining solution (1° AS) (see Recipes)
5 mL secondary antibody staining solution (2° AS) (see Recipes)
10 mL DNA stain solution (DSS) (see Recipes)
10 mL whole protein staining solution (WPSS) (see Recipes)
Embedding solution (ES) (see Recipes)
Equipment
Microm HM355S (Thermo Scientific, catalog number: 90 520 0STS)
Steamer (Betty Bossi, catalog number: 10003614)
(Optional: to ease liquid handling steps) Washer dispenser (Agilent, catalog number: EL406)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Kramer, B. A., Sarabia del Castillo, J., Pelkmans, L. and Gut, G. (2023). Iterative Indirect Immunofluorescence Imaging (4i) on Adherent Cells and Tissue Sections. Bio-protocol 13(13): e4712. DOI: 10.21769/BioProtoc.4712.
- Kramer, B. A., Sarabia Del Castillo, J. and Pelkmans, L. (2022). Multimodal perception links cellular state to decision-making in single cells. Science 377(6606): 642-648.
分类
细胞生物学 > 细胞成像 > 固定细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link