- EN - English
- CN - 中文
A New Methodology for the Quantification of Neutrophil Extracellular Traps in Patient Plasma
定量患者血浆中中性粒细胞外陷阱的新方法
发布: 2023年06月20日第13卷第12期 DOI: 10.21769/BioProtoc.4701 浏览次数: 3118
评审: Alessandro DidonnaKomuraiah MyakalaMarco Di Gioia
Abstract
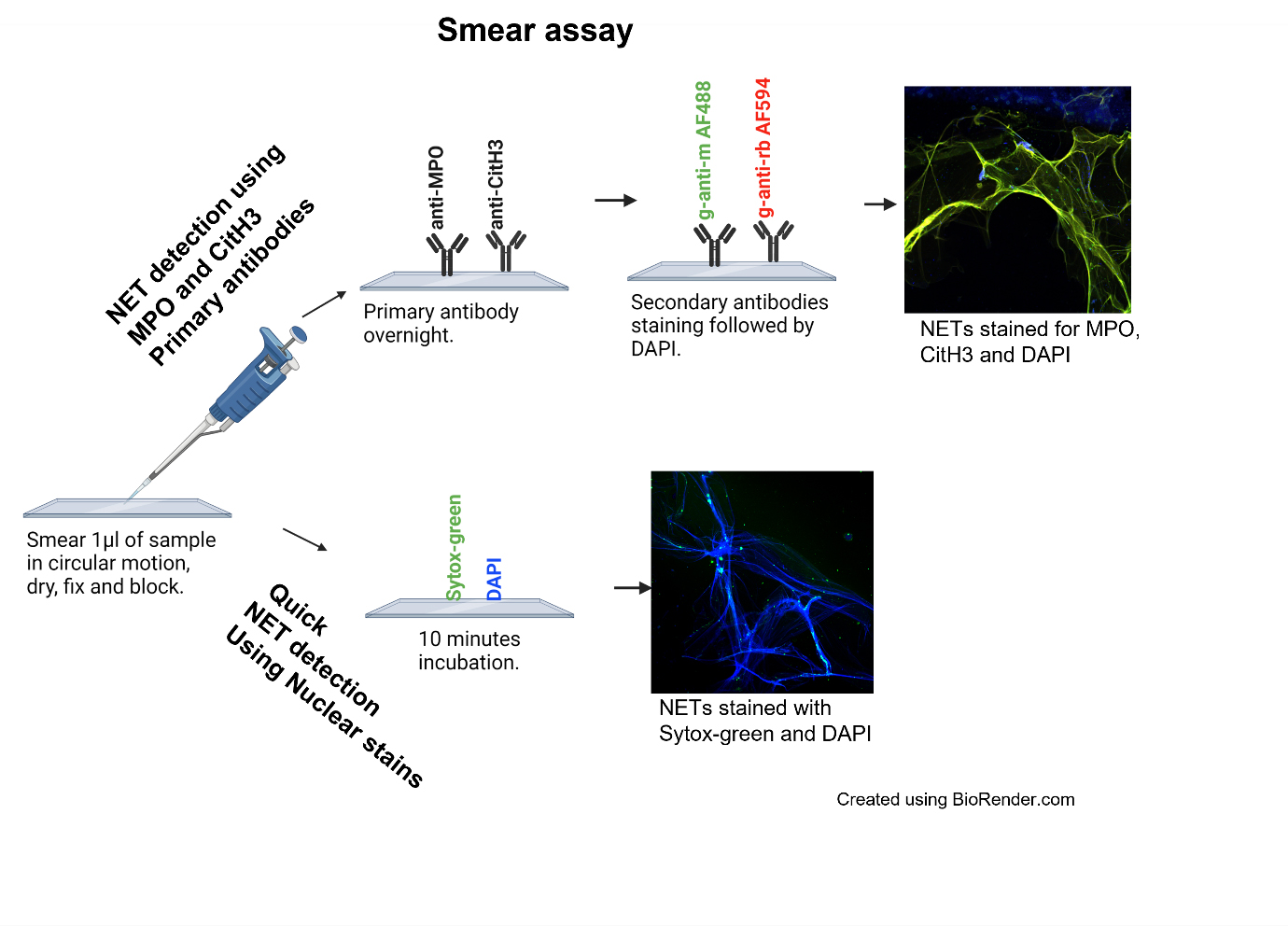
Neutrophil extracellular traps (NETs) are web-like structures made up of decondensed chromatin fibers along with neutrophil granular proteins that are extruded by neutrophils after activation or in response to foreign microorganisms. NETs have been associated with autoimmune and inflammatory diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis, coronavirus disease 2019 (COVID-19), and others. There are reliable methods available to quantitate NETs from neutrophils, but their accurate quantification in patient plasma or serum remains a challenge. We developed a highly sensitive ELISA to detect NETs in serum/plasma and designed a novel smear immunofluorescence assay to detect NETs in as little as 1 μL of serum/plasma. We further validated our technology on plasma samples from SLE patients and healthy donors that carry interferon regulatory factor 5 genetic risk. The multiplex ELISA combines the use of three antibodies against myeloperoxidase (MPO), citrullinated histone H3 (CitH3), and DNA to detect the NET complexes with higher specificities. The immunofluorescence smear assay can visually detect intact structures of NETs in 1 μL of serum/plasma and provide similar results that correlate with findings from the multiplex ELISA. Furthermore, the smear assay is a relatively simple, inexpensive, and quantifiable method of NET detection for small volumes.
Graphical overview
Background
Neutrophils are the most abundant leukocyte in the human blood and are the first cells to respond to an external stimulus such as a pathogen and/or inflammatory trigger (Kaplan and Radic, 2012; Matta et al., 2022). They migrate to the site of infection where they protect the host by phagocytosing, killing, and digesting pathogens (Kaplan and Radic, 2012; Matta et al., 2022). Another powerful tool neutrophils use to defend the body is the release of neutrophil extracellular traps (NETs). NETs can trap microorganisms and kill them by releasing granular antimicrobial peptides and proteins such as neutrophil elastase (NE), myeloperoxidase (MPO), and cathepsin G(3); however, when this process gets dysregulated or NET clearance is diminished, it can lead to tissue damage, autoimmunity, and other inflammatory conditions (Lande et al., 2011; Kaplan and Radic, 2012; Corsiero et al., 2016; Bruschi et al., 2020; Matta et al., 2022).
Formation of NETs involves the citrullination of histones, chromatin decondensation, and disintegration of the nuclear membrane followed by the release of nuclear content along with proteins such as MPO and NE (Kaplan and Radic, 2012; Matta et al., 2022). Traditionally, NETs are detected by using either anti-MPO or anti-citrullinated histone H3 (CitH3), or neutrophil elastase as coating antibody separately followed by anti-DNA detecting antibody (Zuo et al., 2020 and 2021). We have developed and validated a more sensitive ELISA, where we use a cocktail of anti-MPO and anti-CitH3 antibodies as coating antibodies, followed by anti-DNA as the detecting antibody. We further optimized the best blocking and substrate combination suited for increased sensitivity for ELISA. To the best of our knowledge, we are the first one to develop the smear assay to visualize NETs in small amounts of patient plasma. This method requires minimal time, reagents, specialized equipment, and/or cost. These techniques can be applied to detection of NETs in serum/plasma of patients with autoimmune diseases and other inflammatory conditions where NETs have been shown to play a crucial role. But apart from that, we propose that the application of these techniques can be extended to other bodily fluids as well, where accumulation of NETs has been shown to play a role in disease pathogenesis. For example, NETs can be quantified in the synovial fluid of joints to study rheumatoid arthritis (Corsiero et al., 2016) or excretion of NETs in urine in certain inflammatory conditions such as urinary tract infections (Yu et al., 2019). Since the smear assay requires only 1 μL of sample, this assay can be applied to samples that can be obtained only in smaller quantities; for example, NETs have shown to be involved in many ocular diseases such as glaucoma, age-related macular degeneration, dry eye, and diabetic retinopathy for which only a very small volume of ocular fluid can be obtained (Martínez-Alberquilla et al., 2022). Another example is bronchiolar lavage, where NETs have been shown to be elevated in patients with pneumonia-related acute respiratory distress syndrome (Bendib et al., 2019), primary graft dysfunction after lung transplantation (Sayah et al., 2015), and COVID-19 (Yaqinuddin et al., 2020). Moreover, these techniques can also be applied to pediatric autoimmune and inflammatory diseases such as pediatric lupus, where there are limitations on sample volume (Garcia-Romo et al., 2011).
Materials and reagents
Poly-L-Lysine adhesive microscope slides (Newcomer Supply, catalog number: 5010)
ImmulonTM MicrotiterTM 96-well plates (Thermo Scientific, catalog number: 3855)
Mouse monoclonal antibody [2C7] to myeloperoxidase (MPO) (Abcam, catalog number: 25989)
Rabbit polyclonal antibody to histone H3 (anti-CitH3 citrulline R2 + R8 + R17) (Abcam, catalog number: 5103)
Sytox Green nucleic acid stain (Invitrogen, catalog number: S7020)
Cell Death Detection ELISA PLUS Anti-DNA POD kit (Roche, catalog number: 11774425001)
Goat anti-mouse Alexa Fluor 488 (2 mg/mL) (Invitrogen, catalog number: A11029)
Goat anti-rabbit Alexa Fluor 594 (2 mg/mL) (Molecular Probes, catalog number: A11012)
DAPI (4’,6-Diamidino-2-Phenylindole, dihydrochloride) (Sigma-Aldrich, catalog number: D9542-1MG)
TMB (3,3’,5,5’-tetramethylbenzidine) enhanced one component HRP membrane substrate (Sigma-Aldrich, catalog number: T9455)
2N sulfuric acid stop solution (Reagents, catalog number: CS106300-500A)
Normal rat serum control (Fisher Scientific, catalog number: 10-710-C)
Tween 20 (Thermo Fisher, catalog number: J20605.AP)
Triton X-100 (Thermo Fisher, catalog number: HFH10)
Bovine serum albumin (BSA) (Fisher catalog number: BP9703-100)
Formaldehyde (Sigma, catalog number: 252549)
Na2CO3 (Sigma, catalog number: 497-19-8)
NaHCO3 (Sigma, catalog number: 144-55-8)
VectaMount AQ mounting medium (Vector Labs, catalog number: H-5501)
Dulbecco’s Phosphate Buffer saline (PBS without calcium and magnesium chloride (Sigma, catalog number: D8537-500ML)
Wash buffer (see Recipes)
Blocking buffer (see Recipes)
Dilution buffer (see Recipes)
Coating buffer (see Recipes)
Smear dilution buffer (see Recipes)
Smear blocking buffer (see Recipes)
Fixing buffer (see Recipes)
Equipment
Pipettes, multi-channel pipettes for washings and tips
Synergy Neo2 Multi-Mode microplate reader (BioTek, model: BTNEO2)
EVOS M7000 imaging system (Invitrogen, catalog number: AMF7000)
ZEISS Confocal LSM 880 Airyscan
NanoDrop 2000 (Thermo Fisher Scientific, catalog number: ND-2000)
Software
ImageJ—Java-based program (V1.8) (https://imagej.nih.gov/ij/download.html)
GraphPad Prism 8 (https://www.graphpad.com/scientific-software/prism/)
MyAssays online program (https://www.myassays.com/download-and-install-myassays-desktop.html)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Matta, B., Battaglia, J. and Barnes, B. J. (2023). A New Methodology for the Quantification of Neutrophil Extracellular Traps in Patient Plasma. Bio-protocol 13(12): e4701. DOI: 10.21769/BioProtoc.4701.
分类
免疫学 > 免疫细胞功能 > 嗜中性粒细胞
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link