- EN - English
- CN - 中文
Measurement of Total Phosphorus and Polyphosphate in Chlamydomonas reinhardtii
莱茵衣藻中总磷和多磷酸盐的测定
(*contributed equally to this work) 发布: 2023年06月05日第13卷第11期 DOI: 10.21769/BioProtoc.4692 浏览次数: 1466
评审: Ansul LokdarshiAnonymous reviewer(s)

相关实验方案

一种利用 TMSD 甲基化结合 LC-MS 分析法快速定量细胞培养物中长链聚异戊烯类磷酸盐的新方法
Dipali Kale [...] Britta Brügger
2023年11月20日 1505 阅读
Abstract
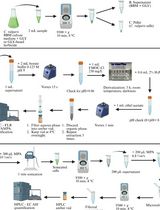
Phosphorus is an essential nutrient for plants. Green algae usually store excess P as polyphosphate (polyP) in the vacuoles. PolyP, a linear chain of three to hundreds of phosphate residues linked by phosphoanhydride bonds, is important for cell growth. Based on the previous method of polyP purification with silica gel columns (Werner et al., 2005; Canadell et al., 2016) in yeast cells, we developed a protocol to purify and determine the total P and polyP in Chlamydomonas reinhardtii by a quick, simplified, and quantitative method. We use hydrochloric acid or nitric acid to digest polyP or total P in dried cells and analyze P content using the malachite green colorimetric method. This method may be applied to other microalgae.
Keywords: Green algae (绿藻)Background
As an essential nutrient, phosphorus is required for the constitution of cellular components such as nucleic acids, phospholipids, and ATP. P is also required for the regulation of protein phosphorylation in plants. P is stored as inorganic phosphate (Pi) in the vacuoles of land plants and as inorganic polyphosphate (polyP) in chlorophyte algae. PolyP is a linear chain composed of several phosphate residues that are linked together by phosphoanhydride bonds. PolyP is an important metabolite and a signaling molecule acting as an energy source, a regulator of gene expression, a channel-forming component, and a storage form of Pi found in green plants (Wang et al., 2021).
Since the polyP is a mixture with different chain lengths, few analytical methods are currently available for polyP quantitation. In order to determine the content of polyP, it usually has to be degraded into Pi first, and then its content can be estimated by measuring the amount of Pi released. Two experimental methods are widely used for quantitatively estimating the amount of polyP in vitro (Aschar-Sobbi et al., 2008). The first one is based on the hydrolysis of polyP by hydrochloric acid and subsequent measurement of the Pi released. The second one is based on the activity of two enzymes, exopolyphosphatase and polyphosphate kinase, which can digest and convert the polyP into Pi for measurement. However, the expression and purification of these enzymes are complicated and time consuming (Christ et al., 2020).
Here, we describe an updated protocol for quantitative measurement of the polyP content in algae. We use silica gel columns to purify polyP and then convert it into Pi through digestion by hydrochloric acid. Although this method is not very accurate, because some short-chain-length polyP could not be completely extracted by the silica gel columns, it is economical and convenient. This protocol is relatively simple and time saving, leading to a fast determination of the polyP in Chlamydomonas, and should be considered suitable for polyP determination in other microalgae.
Materials and reagents
Centrifuge tubes (1.5, 2, 15, and 50 mL) (Sangon Biotech)
Sterile culture flasks (50 and 250 mL) (Sangon Biotech)
Pipettes (10, 20 and 200 μL, 1 and 5 mL) (Eppendorf)
96-well ELISA plate (Sangon Biotech, catalog number: F605031)
Petri dishes (Sangon Biotech, catalog number: F611003)
47 mm filter paper sheets (Whatman, catalog number: 1822047)
Spin Columns CA2 from TIANgel Midi Purification kit (TIANGEN, catalog number: DP209)
Hydrochloric acid (HCl)
Nitric acid (HNO3)
98% sulfuric acid (H2SO4)
Tris (C4H11NO3) (Sangon Biotech, catalog number: A100826)
Glacial acetic acid (CH3COOH) (Sangon Biotech, catalog number: A501931)
Ammonium chloride (NH4Cl) (Sangon Biotech, catalog number: A501569)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (Sangon Biotech, catalog number: A610329)
Calcium chloride dihydrate (CaCl2·2H2O) (Sangon Biotech, catalog number: A610050)
Dipotassium hydrogen phosphate (K2HPO4) (Sangon Biotech, catalog number: A610447)
Potassium phosphate monobasic (KH2PO4) (Sangon Biotech, catalog number: A600445)
EDTA disodium salt (C10H14N2O8Na2·2H2O) (Sangon Biotech, catalog number: A100105)
Zinc sulfate heptahydrate (ZnSO4·7H2O) (Sangon Biotech, catalog number: A602906)
Iron(II) sulfate heptahydrate (FeSO4·7H2O) (Sangon Biotech, catalog number: A600461)
Boric acid (H3BO3) (Sangon Biotech, catalog number: A100588)
Manganese (II) chloride tetrahydrate (MnCl2·4H2O) (Sangon Biotech, catalog number: A500331)
Cobalt chloride hexahydrate (CoCl2·6H2O) (Sigma-Aldrich: catalog number: 255599)
Copper(II) sulfate pentahydrate (CuSO4·5H2O) (Sangon Biotech, catalog number: A600063)
Ethanol absolute (Sangon Biotech, catalog number: A500737)
Neutral red (Sangon Biotech, catalog number: A600895)
1 M Tris-HCl (pH 7.5) (Sangon Biotech, catalog number: A610283)
Sodium iodide (NaI) (Sangon Biotech, catalog number: A610283)
Ammonium heptamolybdate tetrahydrate [(NH4)6Mo7O24·4H2O] (Sangon Biotech, catalog number: A600067)
Malachite green oxalate salt (Sangon Biotech, catalog number: A620330)
TAP medium (see Recipes)
40× TAP salt mixture (see Recipes)
Phosphate solution (50 μg/mL) (see Recipes)
Hutner's trace elements (see Recipes)
TAP solid medium (see Recipes)
1 M sulfuric acid (H2SO4) (see Recipes)
2 M NaOH (see Recipes)
70% ethanol (see Recipes)
0.1% neutral red solution (w/v) (see Recipes)
1 M Tris-HCl (pH 7.5) supplemented to 6% (v/v) with 0.1% neutral red solution (w/v) (see Recipes)
6 M NaI (see Recipes)
Wash buffer (see Recipes)
2 M hydrochloric acid (HCl) (see Recipes)
Phosphate (Pi) solution (10 μg/mL) (see Recipes)
28 mM ammonium heptamolybdate in 2.1 M H2SO4 (see Recipes)
0.76 mM malachite green in 0.35% polyvinyl alcohol (see Recipes)
Equipment
Spectrophotometer (Shimadzu, model: UVmini-1240)
Centrifuge (Thermo Scientific, catalog number: 75002411)
Hitachi Himac CT15RE tabletop high-speed microcentrifuge
Hitachi CR21N high-speed refrigerated centrifuge
Vortex (IKA, model: IKA MS 3 basic)
Thermal cycler (Thermo Scientific, catalog number: 4484073)
Microplate reader (Tecan, model: Infinite® F50 microplate reader)
Vacuum pump (JinTeng, model: GM-0.5B)
Analytical balance (Sangon Biotech, catalog number: G001142)
Air dry oven (Sangon Biotech, catalog number: G003409)
Clean bench (AIRTECH, catalog number: SW-CJ-2F)
Fume hood (ALPHA, VAV controller)
Nikon Digital Camera D7200
Software
Microsoft Excel (Microsoft, Inc.)
Microsoft PowerPoint (Microsoft, Inc.)
Prism GraphPad (Dotmatic, Inc.)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yang, Y., Ren, S., Jia, X., Zeng, H., Wang, L., Zhu, Y. and Yi, K. (2023). Measurement of Total Phosphorus and Polyphosphate in Chlamydomonas reinhardtii. Bio-protocol 13(11): e4692. DOI: 10.21769/BioProtoc.4692.
分类
植物科学 > 植物生物化学 > 其它化合物
生物化学 > 其它化合物 > 多磷酸盐
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link