- EN - English
- CN - 中文
Determination of Interleukin-17A and Interferon-γ Production in γδ, CD4+, and CD8+ T Cells Isolated from Murine Lymphoid Organs, Perivascular Adipose Tissue, Kidney, and Lung
从小鼠淋巴器官、血管周围脂肪组织、肾脏和肺分离的γδ、CD4+和CD8+T细胞中的白细胞介素-17A和干扰素-γ产生的测定
发布: 2023年05月20日第13卷第10期 DOI: 10.21769/BioProtoc.4679 浏览次数: 3634
评审: Meenal SinhaJulie WeidnerClara Lubeseder-MartellatoAnonymous reviewer(s)

相关实验方案
Cluster FLISA——用于比较不同细胞系蛋白表达效率及蛋白亚基聚集状态的方法
Sabrina Brockmöller and Lara Maria Molitor
2025年11月05日 1206 阅读
Abstract
T cells localized to the kidneys and vasculature/perivascular adipose tissue (PVAT) play an important role in hypertension and vascular injury. CD4+, CD8+, and γδ T-cell subtypes are programmed to produce interleukin (IL)-17 or interferon-γ (IFNγ), and naïve T cells can be induced to produce IL-17 via the IL-23 receptor. Importantly, both IL-17 and IFNγ have been demonstrated to contribute to hypertension. Therefore, profiling cytokine-producing T-cell subtypes in tissues relevant to hypertension provides useful information regarding immune activation. Here, we describe a protocol to obtain single-cell suspensions from the spleen, mesenteric lymph nodes, mesenteric vessels and PVAT, lungs, and kidneys, and profile IL-17A- and IFNγ-producing T cells using flow cytometry. This protocol is different from cytokine assays such as ELISA or ELISpot in that no prior cell sorting is required, and various T-cell subsets can be identified and individually assessed for cytokine production simultaneously within an individual sample. This is advantageous as sample processing is kept to a minimum, yet many tissues and T-cell subsets can be screened for cytokine production in a single experiment. In brief, single-cell suspensions are activated in vitro with phorbol 12-myristate 13-acetate (PMA) and ionomycin, and Golgi cytokine export is inhibited with monensin. Cells are then stained for viability and extracellular marker expression. They are then fixed and permeabilized with paraformaldehyde and saponin. Finally, antibodies against IL-17 and IFNγ are incubated with the cell suspensions to report cytokine production. T-cell cytokine production and marker expression is then determined by running samples on a flow cytometer. While other groups have published methods to perform T-cell intracellular cytokine staining for flow cytometry, this protocol is the first to describe a highly reproducible method to activate, phenotype, and determine cytokine production by CD4, CD8, and γδ T cells isolated from PVAT. Additionally, this protocol can be easily modified to investigate other intracellular and extracellular markers of interest, allowing for efficient T-cell phenotyping.
Keywords: T-cell activation (T细胞活化)Background
Adaptive immune cells, including CD4+, CD8+, and γδ T cells, play an important role in hypertension and vascular injury (Caillon et al., 2019; Higaki et al., 2019; Higaki et al., 2021). T-cell subtypes are programmed to produce interleukin-17 (IL-17) or interferon-γ (IFNγ). Naïve T cells can also be induced to produce IL-17 through stimulation of the IL-23 receptor. Hypertension is associated with increased infiltration of T cells in perivascular adipose tissue (Caillon et al., 2017), with both IL-17 and IFNγ having been demonstrated to contribute to hypertension (Madhur et al., 2010; Itani et al., 2016). We have therefore developed a protocol to profile CD4+, CD8+, and γδ T-cell subtypes producing IL-17A and/or IFNγ in tissues relevant to hypertension. This is accomplished by performing intracellular staining with fluorescent-labeled monoclonal antibodies and flow cytometry. This protocol was used to investigate the role of the IL-23 receptor in the regulation and homeostasis of IL-17A-producing γδ T cells, specifically those localized to mesenteric vessels and perivascular adipose tissue (MV/PVAT) in an angiotensin II–induced model of hypertension (Shokoples et al., 2022). We showed that IL-23 receptor deficiency caused a reduction in IL-17A-producing γδ T cells and expansion of IFNγ-producing CD4+, CD8+, and γδ T cells. Angiotensin II treatment led to expansion of IFNγ-producing CD4+, CD8+, and γδ T cells in IL-23 receptor knockout mice. However, only IFNγ-producing γδ T cells expanded in angiotensin II–treated wild-type mice. Accordingly, profiling cytokine-producing T-cell subtypes in tissues relevant to hypertension provides useful information regarding immune activation.
Here, we describe methods used to obtain single-cell suspension from spleens, mesenteric lymph nodes (mLNs), MV/PVAT, lungs, and kidneys from four wild-type C57BL/6 mice. This is followed by an in vitro T-cell activation in the presence of the Golgi blocker monensin, which allows for signal amplification by trapping cytokines within activated cells. Next, cells are stained for viability and surface marker expression, which is then followed by IL-17A and IFNγ intracellular staining. Finally, IL-17A- and IFNγ-producing CD4+, CD8+, and γδ T cells are profiled by flow cytometry. This protocol has been extensively tested and optimized for use with samples derived from male C57BL/6 mice, but it should work equally well with other mouse strains as well as female mice. However, some optimization may be required. It is important to note that this technique cannot be used to quantify the cytokine produced, which may represent a limitation. However, this protocol can precisely identify cytokine-producing cells within less numerous T-cell subsets, as there is no requirement for cell sorting before the activation step, so sample loss is minimized. The methods presented in this protocol are advantageous as they provide the ability to screen several tissues for cytokine-producing T cells simultaneously, which can then be followed up with quantitative methods in subsequent experiments.
The methods described below are designed for processing four mice by one person. Someone not experienced in these techniques should start with two animals per day. It is also possible to scale up this protocol by getting help from other colleagues to collect tissues and prepare single-cell suspensions. The remaining part of the protocol can easily be handled by one experienced person. The fluorochrome-conjugated monoclonal antibodies used to determine surface marker expression can be tailored to the individual experiment, as this protocol only contains the minimum number of markers required to phenotype IL-17A and IFNγ production in T cells. This protocol also works when using primary and secondary antibodies to investigate surface marker expression. The protocol is optimized to look at the production of IL-17A and IFNγ in T cells and may need to be modified to investigate the production of other cytokines and/or the expression of other intracellular markers.
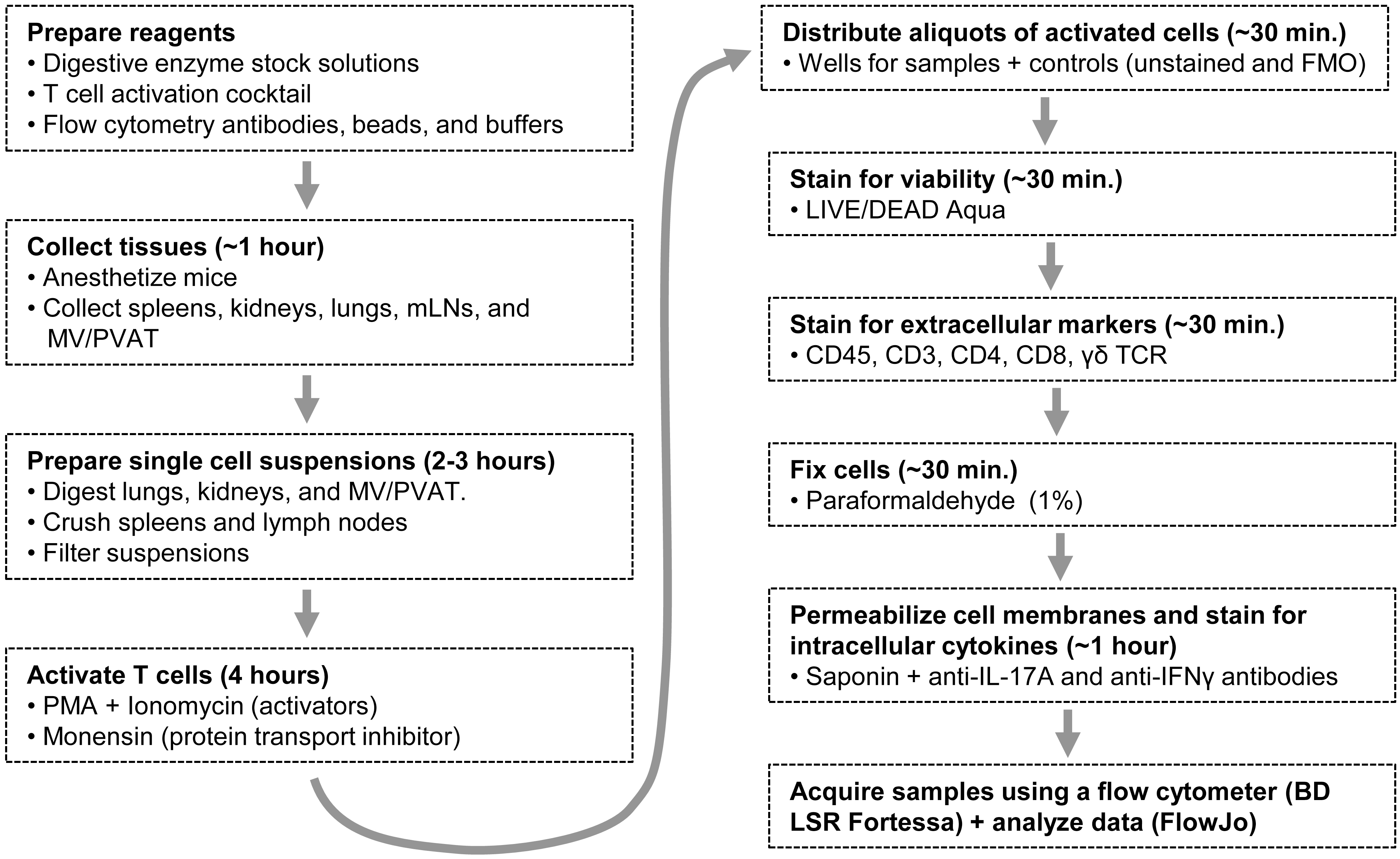
Figure 1. A flow diagram outlining the whole protocol. mLNs: mesenteric lymph nodes; MV/PVAT: mesenteric vessels and perivascular adipose tissue; PMA: phorbol 12-myristate 13-acetate; FMO: Fluorescence Minus One.
Materials and Reagents
Ethyl alcohol anhydrous, USP (Commercial alcohols by Greenfield Global, catalog number: P016EAAN)
Double-distilled water
Centrifuge tubes, 50 mL (VWR, catalog number: 89039-656)
Sterile 100 mm tissue culture dishes, standard (Sarstedt, catalog number: 83.3902)
Cell strainers, 70 μm nylon mesh (Sarstedt, catalog number: 83.3945.070)
Tuberculin syringes, 1 mL with luer slip tip (BD Biosciences, catalog number: BD309659)
Luer lock syringes, 3 mL (BD Biosciences, catalog number: BD309657)
Falcon serological pipette, 10 mL (VWR, catalog number: CA53300-523)
Pipette tips: 10 μL for Gilson PIPETMAN Classic P2 (Sarstedt, catalog numbers: 70.3010 for bag and 70.3010.200 for racked tips), 200 μL for Gilson PIPETMAN Classic P20 and P200 (Gilson, catalog number 70.3030 for bag and 70.3050.200 for racked tips), and 1,000 μL for Gilson PIPETMAN Classic P1000 (Gilson, catalog number 70.3050 for bag and 70.3050.200 for racked tips); 250 μL pipette tips RC LTS used for Rainin multichannel pipette (METTLER TOLEDO, catalog number: 17001118 for bag and 30389243 for racked tips)
Falcon 5 mL polystyrene round-bottom tubes (VWR, catalog number: 352058)
Racked mini tubes, 1.1 mL (VWR, catalog number: 89005-572)
Microtubes, 1.5 mL with conical base and attached flat cap (Sarstedt, catalog number: 72.690.300)
Microtubes, 5 mL with conical base and attached flat cap (Sarstedt, catalog number: 72.701.400)
Sterile Falcon 24-well tissue culture plates, flat bottom with lid (VWR, catalog number: 353047)
Nunc 96-well polystyrene conical bottom microwell plates (Thermo Fisher Scientific, catalog number: 249570)
Isoflurane (CDMV, catalog number: 55868225)
Oxygen gas (O2)
Surgical sutures for small animal surgery, non-absorbable silk 7-0 (Braintree Scientific, catalog number: SUT-S 103)
Phosphate buffered saline (PBS) pH 7.4, Ca2+ and Mg2+ free, 500 mL (Thermo Fisher Scientific, catalog number: 10010023)
Roswell Park Memorial Institute 1640 (RPMI), with L-glutamine and phenol red, HEPES-free (Thermo Fisher Scientific, catalog number: 11875093)
Collagenase A from Clostridium histolyticum, lyophilized, ≥0.15 U/mg protein (Roche, catalog number: 10103586001)
Collagenase type 2, lyophilized, >125 U/mg protein (Worthington Biochemical, catalog number: LS004176)
Elastase, lyophilized, ≥3 U/mg protein (Worthington Biochemical, catalog number: LS002292)
Hyaluronidase, lyophilized, ≥300 U/mg protein (Sigma-Aldrich, catalog number: H2126-500MG)
Trypsin inhibitor, soybean, purified (Worthington Biochemical, catalog number: LS003570)
Phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich, catalog number: P1585-1MG)
Ionomycin (Sigma-Aldrich, catalog number: I0634-1MG)
Monensin 1,000× solution (BioLegend, catalog number: 420701). Monensin solution is supplied by BioLegend as a 1,000× working solution in 70% ethanol. The concentration is 2 mM. Store the monensin 1,000× solution at 4 °C until used
LIVE/DEAD Fixable Aqua Dead Cell Stain kit (405 nm excitation, 200 assays) (Thermo Fisher Scientific, catalog number: L34957). The kit contains five vials of stain (component A) and one vial of anhydrous dimethyl sulfoxide (DMSO, component B)
Fetal bovine serum (FBS), qualified, Canada origin, 500 mL (Thermo Fisher Scientific, catalog number: 12483020)
Mouse Fc blocking reagent: purified rat anti-mouse CD16/CD32 Fc-block (BD Biosciences, catalog number: 553142, clone 2.4G2, 0.5 mg, 0.5 mg/mL)
Antibodies for extracellular and intracellular staining are presented in Table 1
Table 1. Antibodies for extracellular and intracellular staining
Antigen Antibodies Clone, company (catalog #) CD45 BV786-conjugated rat anti-mouse CD45 antibody 30-F11, BD Biosciences (564225) CD3 BUV395-conjugated rat anti-mouse CD3 antibody 17A2, BD Biosciences (740268) CD4 PerCP-eFluor710-conjugated rat anti-mouse CD4 antibody RM4-5, eBioscience (46-0042-82) CD8a AF700-conjugated rat anti-mouse CD8a antibody 53-6.7, BioLegend (100730) TCR γδ PE-CF594-conjugated hamster anti-mouse TCR δ antibody GL3, BD Biosciences (563532) IL-17A APC-conjugated rat anti-mouse IL-17A antibody eBio17B7, eBioscience (17-7177-81) IFNγ AF488-conjugated rat anti-mouse IFNγ antibody XMG1.2, eBioscience (53-7311-82) AF488 and AF700: Alexa-Fluor 488 and 700; APC: allophycocyanin; BUV395: Brilliant Ultraviolet 395; BV786: Brilliant Violet 786; CD: cluster of differentiation; CF594: cyanine‐based fluorescent dye 594; IL-17A: interleukin-17A; IFNγ: interferon gamma; PE: phycoerythrin; PerCP: peridinin-chlorophyll-protein.
Paraformaldehyde 10% aqueous solution (PFA), 10 × 10 mL (Electron Microscopy Sciences, catalog number: 15712)
Probumin® bovine serum albumin (BSA), 100 g, life science grade (Sigma-Aldrich, catalog number: 810683)
Saponin, 100 g (Calbiochem, catalog number: 558255)
InvitrogenTM UltraComp eBeadsTM (Thermo Fisher Scientific, catalog number: 01-2222-42)
ArcTM Amine-Reactive Compensation Bead kit (Thermo Fisher Scientific, catalog number: A10346). The kit contains ArC-reactive beads (Component A) that bind any of the amine-reactive dyes and provide a positive signal, and ArC-negative beads (Component B), which have no reactivity and provide a negative compensation control)
The following reagents and equipment need to be prepared prior to beginning the protocol
Detailed descriptions can be found under Recipes.
Reagents and equipment to prepare in advance (see Recipes)
70% ethanol
Sterile pipette tips
Sterile dissecting tools
Digestion stock solution (8×)
Hyaluronidase stock solution (8×)
PMA 1 mg/mL stock solution
Ionomycin stock solution (1 mg/mL)
LIVE/DEAD stain stock solution
FBS aliquots
Permeabilization buffer
Reagents to prepare within three days of the experiment (see Recipes)
Pre-diluted antibody mixtures
Reagents to prepare on the day of the experiment (see Recipes)
24-well tissue culture plate for the collection of tissues
Labeled 50 mL centrifuge tube for collecting the intestines and mesenteric bed
T-cell activation cocktail (1 mL/sample)
PFA 1% solution
LIVE/DEAD staining solution
Equipment
Small animal isoflurane anesthesia machine with adequate gas scavenging system or filter, an induction chamber constructed of a see-through material (glass, polycarbonate, etc.), and a mouse anesthesia nosecone or mask (Dispomed)
Necropsy tools
1× surgical scissors, sharp-blunt, 14.5 cm (Fine Science Tools, catalog number: 14001-14)
1× fine scissors, sharp, 9 cm (Fine Science Tools, catalog number: 14061-09)
1× delicate suture tying forceps, 9 cm, tip: angled 45° and smooth (Fine Science Tools, catalog number: 11063-07)
2× Graefe forceps, 10 cm, tip: curved and serrated (Fine Science Tools, catalog number: 11051-10)
Dissecting tools
2× splinter forceps (tweezers), number 5 long points, 11 cm (Almedic, catalog number: A10-704)
1× student Vannas spring scissors, tips: sharp, cutting edge: 5 mm, tip diameter: 0.35 mm, length: 9 cm (Fine Science Tools, catalog number: 91500-09)
Biological safety cabinet, class II (Thermo Fischer Scientific)
Drummond Portable Pipet Aid XP (Cole-Parmer)
Single-edged surgical carbon-steel blades (Thermo Fisher Scientific, catalog number: S17302)
Ice packs stored in a -20 °C freezer
Binocular dissecting microscope Leica MS 5 with variable magnification (Leica Microsystems)
VWR Boekel hybridization incubator oven at 37 °C equipped with a rocker platform (VWR)
Cell culture incubator (5% CO2, 37 °C, Thermo Fischer Scientific)
Refrigerator at 4 °C (Thermo Fisher Scientific)
Dissection Petri dish: 100 × 10 mm Pyrex Petri dish (Cole-Parmer, catalog number: UZ-34551-02) with wax bottom lining (Thermo Fisher Scientific, catalog number: S80769) for pinning down samples
BD PrecisionGlide needle 26 G × 5/8 Sub-1 (BD, catalog number: 305115) for pinning down the mesenteric bed in the dissection Petri dish
Centrifuge 5810 R with swinging bucket rotor A-4-81 and adaptors for 96-well plates and 5 and 50 mL Eppendorf tubes (Eppendorf)
P2, P20, P200, and P1000 Gilson PIPETMAN Classic single-channel pipettors (Thermo Fisher)
Rainin Pipet-Lite L200 8-Channel Multichannel 20–200 μL LTS Pipette (METTLER TOLEDO, see Note 1)
Multicolour flow cytometer: BD LSR Fortessa (BD Biosciences), equipped with 355, 405, 488, 561, and 640 nm excitation lasers and appropriate bandpass filters
Software
FlowJo V10 (FlowJo LLC, BD, https://www.flowjo.com)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Comeau, K., Caillon, A., Paradis, P. and Schiffrin, E. L. (2023). Determination of Interleukin-17A and Interferon-γ Production in γδ, CD4+, and CD8+ T Cells Isolated from Murine Lymphoid Organs, Perivascular Adipose Tissue, Kidney, and Lung. Bio-protocol 13(10): e4679. DOI: 10.21769/BioProtoc.4679.
分类
免疫学 > 免疫细胞分离 > 骨髓细胞
生物化学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link