- EN - English
- CN - 中文
Analysis of RNA Polymerase II Chromatin Binding by Flow Cytometry
用流式细胞仪分析RNA聚合酶II的染色质结合情况
发布: 2023年04月20日第13卷第8期 DOI: 10.21769/BioProtoc.4659 浏览次数: 2275

相关实验方案
外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1435 阅读
Abstract
RNA polymerase II (RNAPII) transcribes DNA into mRNA and thereby plays a critical role in cellular protein production. In addition, RNAPII plays a central role in DNA damage responses. Measurements of RNAPII on chromatin may thus give insight into several essential processes in eukaryotic cells. During transcription, the C-terminal domain of RNAPII becomes post-translationally modified, and phosphorylation on serine 5 and serine 2 can be used as markers for the promoter proximal and productively elongating forms of RNAPII, respectively. Here, we provide a detailed protocol for the detection of chromatin-bound RNAPII and its serine 5– and serine 2–phosphorylated forms in individual human cells through the cell cycle. We have recently shown that this method can be used to study the effects of ultraviolet DNA damage on RNAPII chromatin binding and that it can even be used to reveal new knowledge about the transcription cycle itself. Other commonly used methods to study RNAPII chromatin binding include chromatin immunoprecipitation followed by sequencing or chromatin fractionation followed by western blotting. However, such methods are frequently based on lysates made from a large number of cells, which may mask population heterogeneity, e.g., due to cell cycle phase. With strengths such as single-cell analysis, speed of use, and accurate quantitative readouts, we envision that our flow cytometry method can be widely used as a complementary approach to sequencing-based methods to study effects of different stimuli and inhibitors on RNAPII-mediated transcription.
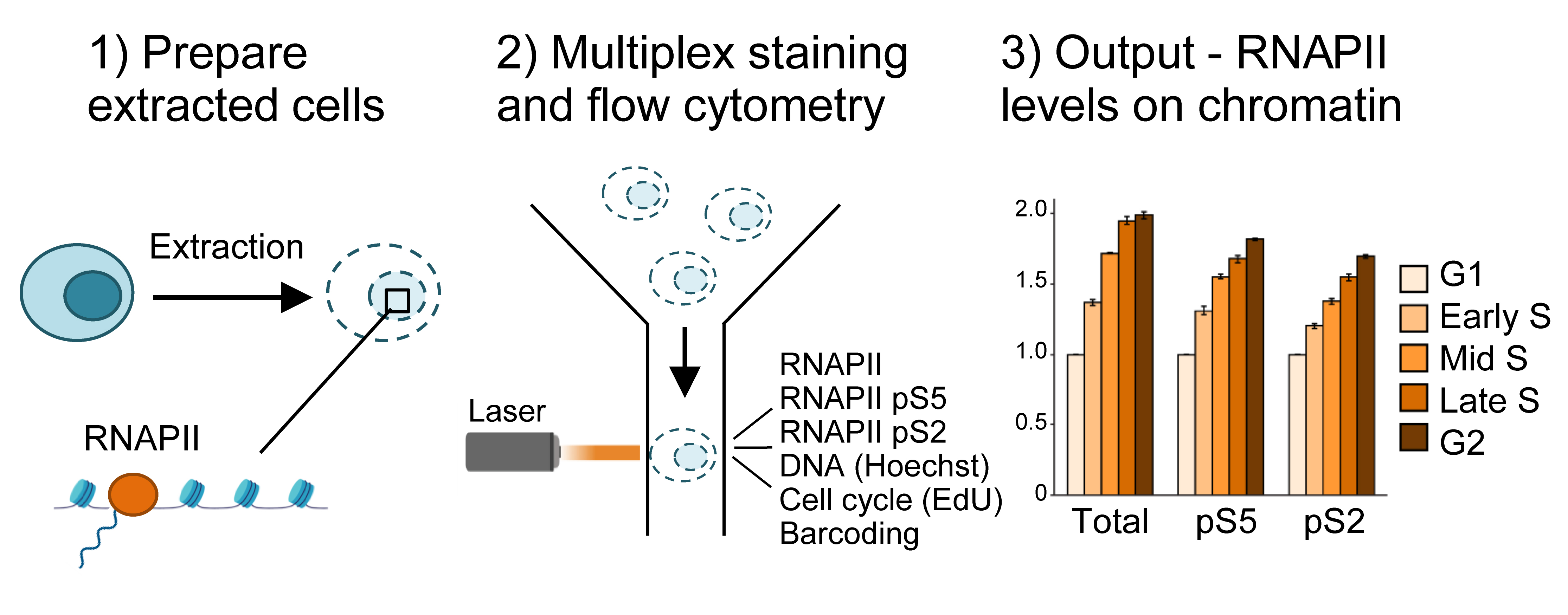
Graphical overview
Background
RNA polymerase II (RNAPII) plays an essential role in protein production by transcribing DNA into mRNA. In addition, it participates in the detection, signaling, and repair after DNA damage (Ljungman and Lane, 2004; Lagerwerf et al., 2011; Lans et al., 2019; Landsverk et al., 2021), and thus also plays a key role in the DNA damage response. Furthermore, RNAPII is essential for cell cycle progression, and is itself regulated by the cell cycle (Enserink and Chymkowitch, 2022). As DNA damage greatly affects the cell cycle by activation of cell cycle checkpoints (Hauge et al., 2021), measuring RNAPII levels on chromatin in individual cell cycle phases is highly relevant both in the absence and presence of DNA damage. Previously, cell cycle effects on RNAPII have been studied, for instance by microscopy (Chan et al., 2012; Teves et al., 2018) and/or chromatin immunoprecipitation/fractionation followed by sequencing (Liang et al., 2015, Wang et al., 2021) or western blotting (Akoulitchev and Reinberg, 1998; Palozola et al., 2017). However, although sequencing or chromatin fractionation techniques can give high resolution sequence information and/or quantitative data, they have so far been based on cell lysates made from a large number of pooled cells. In order to investigate cell cycle effects, these techniques require cell synchronization, which can induce replication stress or changes to transcription. Microscopy offers single-cell resolution and does not require synchronization but is limited in the number of cells analyzed. To overcome these obstacles, we recently described a new method to study RNAPII chromatin binding in single cells through the cell cycle (Bay et al., 2022). This flow cytometry method allows for the determination of cell cycle phases without the need to synchronize cells. Also, thousands of cells are measured per sample per experiment, ensuring accurate quantitative readouts. Using this method on cells treated with the transcriptional inhibitor 5,6-Dichloro-1-beta-Ribo-furanosyl Benzimidazole (DRB), which arrests RNAPII in promoter proximal regions, and the proteasome inhibitor MG132, we showed that promoter proximal RNAPII is degraded under unperturbed conditions, and that this is further enhanced after UV treatment (Bay et al., 2022). In line with our results, degradation of promoter proximal RNAPII after UV treatment was also observed in an independent study by live-cell imaging, chromatin immunoprecipitation followed by sequencing, and western blotting of chromatin fractions (Steurer et al., 2022). Furthermore, using our method, we could observe cell cycle differences in the chromatin loading of RNAPII after UV treatment (Bay et al., 2022). Here, we present the detailed protocol for this method.
A first critical step in the protocol is the cell extraction step, where non-chromatin-bound factors are removed by treatment with mild detergent Triton X-100 (0.5%) prior to fixation (Figure 1). This allows measurements of only chromatin-bound RNAPII. The use of flow cytometry to measure chromatin-bound factors after detergent treatment was first described in the early 90s for the study of chromatin-bound DNA polymerase α (Stokke et al., 1991). Similar assays using extraction prior to fixation have also been used to measure chromatin-binding of other proteins involved in transcription, replication, and repair by flow cytometry (Stokke et al., 1993; Forment et al., 2012; Forment and Jackson, 2015) or immunofluorescence microscopy (Syljuåsen et al., 2005; Britton et al., 2013). Notably, this is the first time such a protocol has been described for RNAPII by flow cytometry. Triton X-100 treatment is performed in a sucrose-containing buffer, similar to pre-extraction/cytoskeletal buffers commonly used in immunofluorescence microscopy (Britton et al., 2013; Kilgas et al., 2021). However, as RNAPII is known to be released from chromatin in mitosis (Parsons and Spencer, 1997), NaCl concentrations were optimized based on the expected low chromatin binding of RNAPII in mitotic cells (Bay et al., 2022). Moreover, as RNAPII is a target for proteasome-mediated degradation (Wilson et al., 2013), particular care should be taken to avoid degradation during cell processing. In this protocol, degradation is prevented by the addition of proteasome inhibitors during extraction, by performing the extraction on ice, and by fixing the cells rapidly after extraction.
Another key feature of the method is multiplexing, i.e., measurement of several different parameters simultaneously in single cells. One advantage of multiplexing is that it allows the incorporation of barcoding into the assay. In this protocol, barcoding is performed by labeling non-treated control cells with an amine-reactive Alexa 647, which binds to intracellular proteins (Krutzik and Nolan, 2006). The resulting Alexa 647–labeled cells are added to all the individual samples prior to staining with antibodies or the EdU Click-iT reaction and can be separated from the sample cells during analysis. The barcoded cells thus provide an internal standard for normalization of the RNAPII signals in the individual samples. This greatly facilitates quantification and accuracy of the assay and enables all the samples to be compared with each other. Multiplexing further permits analysis of RNAPII chromatin levels in specific phases of the cell cycle, by analyzing RNAPII chromatin levels only in cells with a certain DNA and EdU content (Bay et al., 2022).
Choice of antibodies is also critical in this method, as every new antibody used requires validation. The antibodies described here have been validated by us and others (Heidemann et al., 2013; Steurer et al., 2018; Bay et al., 2022), and recognize the N-terminal domain of RNAPII and the serine 5– (pRNAPII S5) and serine 2–phosphorylated (pRNAPII S2) forms of RNAPII. pRNAPII S5 is mostly associated with promoter-proximal regions, and global levels can therefore indicate degree of promoter-proximal paused RNAPII (Bay et al., 2022). pRNAPII S2 can be used as a marker for productive elongation (Bay et al., 2022). Finally, the use of EdU to label replicating cells is an important step to distinguish between G1 and early S phase, as well as between late S and G2. EdU is a nucleotide analogue, which is incorporated into the DNA molecule upon DNA replication. As their DNA content is highly similar, G1 vs. early S phase and G2 vs. late S-phase cells are not distinguishable by measurements of DNA content alone. By including EdU labeling, cells with similar DNA content can be separated in G1 vs. early S or G2 vs. late S based on the presence or absence of EdU incorporation, which marks the replicating (S phase) cells. However, this step may be omitted if such finely separated cell cycle determination is not required. If EdU is omitted, determination of cell cycle phase can be done by DNA content alone to obtain G1, S, and G2 fractions [Figure 2 and Dale Rein et al. (2015)].
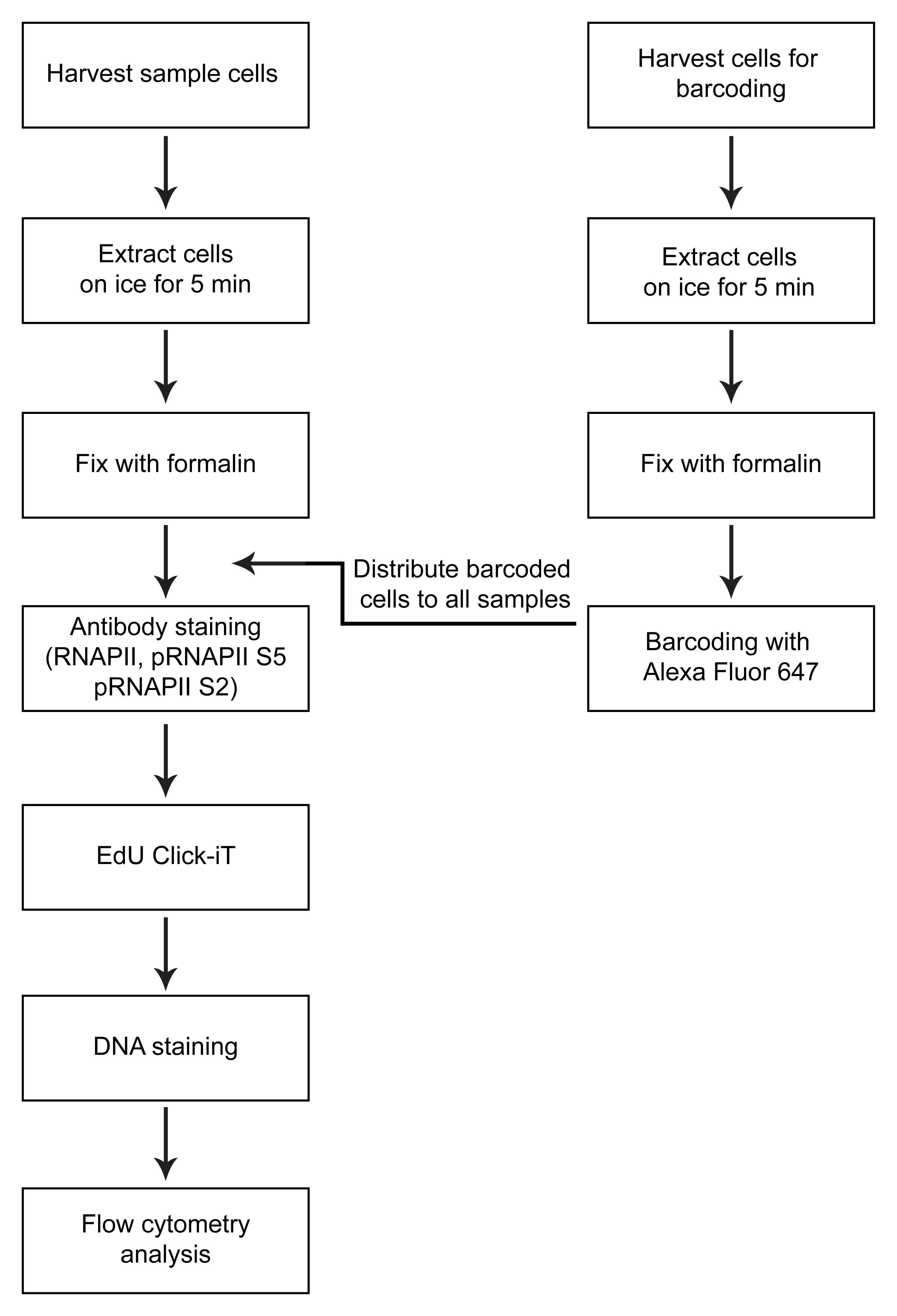
Figure 1. Overview of method
Materials and Reagents
Cell culture and harvest
Serological pipettes, polystyrene
Sterile conical-bottom tubes, 15 mL
Human cervical cancer HeLa Kyoto cells (but other cell types may be used)
Penicillin-Streptomycin (P/S) (Thermo Fisher, catalog number: 15140122)
Fetal bovine serum (FBS) (Biowest, catalog number: S181B-050)
Dulbecco’s modified Eagle medium (DMEM), high glucose, GlutaMAX supplement, pyruvate (Thermo Fisher, catalog number: 31966047)
Culture dishes, 6 cm (VWR, catalog number: 353004)
EdU (1 μM) (Thermo Fisher, catalog number: A10044)
Trypsin 0.25% with EDTA, 100 mL (Life Technologies, catalog number: 25200-056)
Phosphate-buffered saline (10× PBS) (Thermo Fisher, catalog number: 70011-051)
10% formalin solution, neutral-buffered ready-made solution (Sigma-Aldrich, catalog number: HT5011)
Extraction
HEPES (pH 7.9) (Sigma-Aldrich, catalog number: H3784)
MgCl2 (Sigma-Aldrich, catalog number: 208337)
NaCl (Sigma-Aldrich, catalog number: S9888)
Sucrose (Sigma-Aldrich, catalog number: S0389)
Triton X-100 (Sigma-Aldrich, catalog number: T9284-100ML)
PhosSTOP phosphatase inhibitors (Merck, catalog number: 4906837001)
cOmplete protease inhibitor cocktail EDTA-free (Merck, catalog number: 5892791001)
MG132 (Sigma-Aldrich, catalog number: M7449)
ddH2O Milli-Q water
Chromatin extraction buffer (10 mL) (see Recipes)
Staining and preparation of cells for flow cytometry analysis
BD Falcon 5 mL polystyrene with cell strainer cap (VWR, catalog number: 734-0001)
Aluminum foil
Alexa Fluor 647 NHS Ester (Succinimidyl Ester) (Thermo Fisher, catalog number: A20006)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D4540)
Phosphate-buffered saline (10× PBS) (Thermo Fisher, catalog number: 70011-051)
Fetal bovine serum (FBS) (Biowest, catalog number: S181B-050)
Anti-RNAPII (D8L4Y) (Cell Signaling Technology, catalog number: 14958S)
Anti-pRNAPII S5 (3E8) (Sigma-Aldrich, catalog number: 04-1572)
Anti-pRNAPII S2 (3E10) (Sigma-Aldrich, catalog number: 04-1571)
Donkey anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody Alexa Fluor 488 (Thermo Fisher, catalog number: A-21206)
Donkey anti-rat IgG H+L Alexa Fluor 488 pre-adsorbed (Abcam, catalog number: ab150153)
Click-iT Plus EdU Alexa Fluor 594 Flow Cytometry Assay kit (Thermo Fisher, catalog number: C10646)
Bovine serum albumin (BSA) heat-shock fraction, protease free, essentially globulin free, pH 7, ≥98% (Sigma-Aldrich, catalog number: A3059-100G)
Na2HPO4 (Sigma-Aldrich, catalog number: S3264)
KH2PO4 (Sigma-Aldrich, catalog number: P5655)
KCl (Sigma-Aldrich, catalog number: P3911)
NaCl (Sigma-Aldrich, catalog number: S9888)
Ethylenediaminetetraacetic acid (EDTA, pH 7.5)
Igepal CA-630 (Sigma-Aldrich, catalog number: I8896-50ML)
Skim milk powder (Millipore, catalog number: 70166-500G)
DNA-stain Hoechst 33258 (624 μg/mL) (Sigma-Aldrich, catalog number: B1155-25MG)
LX Alexa Fluor 647 NHS Ester (barcoding) (see Recipes)
Flow buffer (1 L) (see Recipes)
Detergent buffer (see Recipes)
Click-iT permeabilization and wash reagent (see Recipes)
Click-iT reaction cocktail (see Recipes)
Equipment
Sterile cell culture hood
37 °C cell culture incubator with CO2 control
Centrifuge (Hettich Rotina 380R Centrifuge, rotor 1754, radius 167 mm)
LSRII flow cytometer (BD Biosciences), Quartz cuvette flow cell, instrument settings can be found in Table 1.
Table 1. LSRII flow cytometer instrument settings
Parameter Laser Band-Pass (nm) Longpass Dichroic filter (nm) Detector Alexa Fluor 488 488 nm–100 mW 525/30 505 PMT B SSC 488 nm–100 mW 488/10 - PMT C FSC 488 nm–100 mW 488/10 - FSC Diode–Silicon photodiode Hoechst 405 nm–100 mW 450/50 - PMT G Alexa Fluor 647 640 nm–40 mW 670/14 - PMT C Alexa Fluor 594 561 nm–45 mW 610/20 600 PMT D
Software
DiVa software is used when obtaining flow cytometry data
FlowJo V10_5 software is used for analyzing the flow cytometry data
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bay, L. T. E., Stokke, T., Syljuåsen, R. G. and Landsverk, H. B. (2023). Analysis of RNA Polymerase II Chromatin Binding by Flow Cytometry. Bio-protocol 13(8): e4659. DOI: 10.21769/BioProtoc.4659.
分类
癌症生物学 > 细胞周期检查点(checkpoint) > 细胞生物学试验
细胞生物学 > 基于细胞的分析方法 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link