- EN - English
- CN - 中文
Imaging Membrane Proteins Using Total Internal Reflection Fluorescence Microscopy (TIRFM) in Mammalian Cells
在哺乳动物细胞中使用全内反射荧光显微镜 (TIRFM) 对膜蛋白进行成像
发布: 2023年02月20日第13卷第4期 DOI: 10.21769/BioProtoc.4614 浏览次数: 1988
评审: Chiara AmbrogioSoumya MoonjelyAnonymous reviewer(s)
Abstract
The cell surfaceome is of vital importance across physiology, developmental biology, and disease states alike. The precise identification of proteins and their regulatory mechanisms at the cell membrane has been challenging and is typically determined using confocal microscopy, two-photon microscopy, or total internal reflection fluorescence microscopy (TIRFM). Of these, TIRFM is the most precise, as it harnesses the generation of a spatially delimited evanescent wave at the interface of two surfaces with distinct refractive indices. The limited penetration of the evanescent wave illuminates a narrow specimen field, which facilitates the localization of fluorescently tagged proteins at the cell membrane but not inside of the cell. In addition to constraining the depth of the image, TIRFM also significantly enhances the signal-to-noise ratio, which is particularly valuable in the study of live cells. Here, we detail a protocol for micromirror TIRFM analysis of optogenetically activated protein kinase C-ϵ in HEK293-T cells, as well as data analysis to demonstrate the translocation of this construct to the cell-surface following optogenetic activation.
Graphic abstract
Background
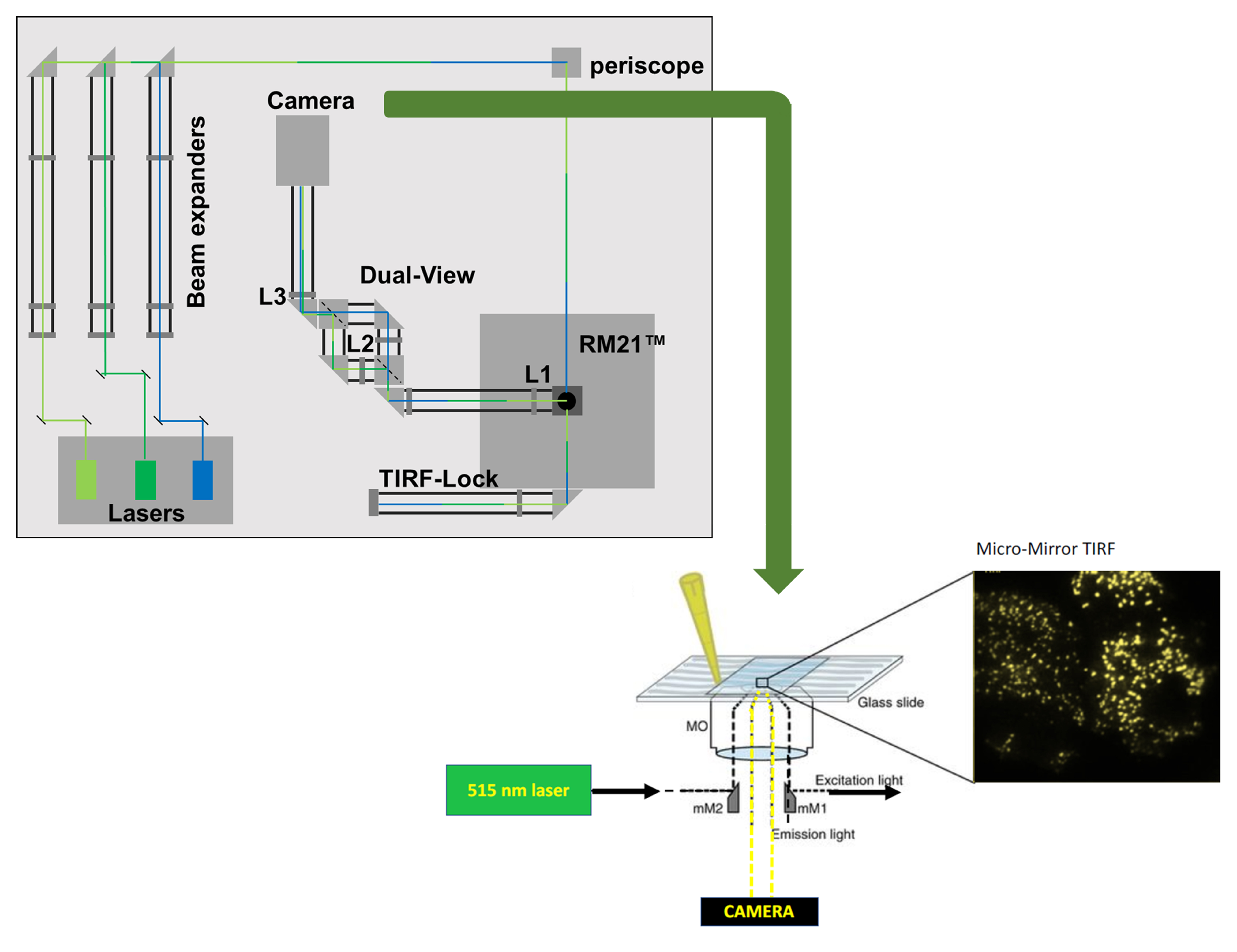
Total internal reflection (TIR) occurs when the angle of incidence of a beam of light upon a refractive surface exceeds a certain threshold, called the critical angle, resulting in reflection of the incident beam of light. While this implies an absence of transmission of light across the interface, there is an escape of electromagnetic energy in the form of an evanescent wave. Total internal reflection fluorescence microscopy (TIRFM) relies on this exponentially decaying evanescent wave that penetrates to a depth of ~100 nm into the sample area. Given that TIR occurs at the interface of two materials with distinct refractive indices, in cell biology experiments the result is that the evanescent wave will only excite fluorescence capable moieties in the evanescent field at or near the cell surface. Thus, TIRFM illuminates fluorescent particles at the cell membrane below the Rayleigh limit for resolution, making the technique well suited for the study of membrane proteins and membrane-delimited signaling events, in fixed or live cell samples.
Several types of microscopy are utilized in the literature to achieve optical sectioning with the intent to localize fluorescent biological moieties to various cellular fractions. Confocal microscopy is a common and versatile technique that has the potential to target any plane of the sample rather than only the glass/liquid interface, as in TIRFM. As with any microscopy, the resolution is determined by the lateral (X-Y) and axial dimensions of the imaging voxel (the Z-plane of a 3-dimensional pixel), each of which is dependent upon numerous factors. Thus, the resolution of the image is determined by the hardware (lens and detector), software, and sample preparation, as well as the fluorophore (protein or dye) and the light source in use. Commonplace confocal microscopes typically operate with a lateral dimension above the Abbe limit of ~250 nm. However, adaptations such as Airy disc scanners and stimulated emission dyes that form the basis of many super resolution techniques can reduce the lateral dimension to below 100 nm. Technologies such as Airy disc confocal, stimulated emission depletion, photoactivated localization microscopy, and stochastic optical reconstruction microscopy have yielded unprecedented information about subcellular architecture and protein localization (Vangindertael et al., 2018). However, they typically require a fixed sample (particularly where specific emission dyes must be employed) and have lower axial resolution (equivalent to the depth of the slice of up to ~500 nm) (Axelrod, 2001) and imaging speeds that are below the biological timescale. The issue of low axial resolution can be improved with two-photon localization microscopy, which, in some cases, can be combined with super resolution in the X-Y plane (Zong et al., 2017). However, this suite of tools is typically expensive and requires specialized hardware, software, and sample preparation. In contrast, TIRFM provides precise localization of fluorescence moieties, at or close to the cell membrane (axial resolution below ~100 nm). In addition to being commercially available, TIRFM systems can be homemade and constructed to image a range of user-preferred fluorophores, including genetically encoded fluorescent proteins, in live cells at relatively high speed in a cost-effective manner. Therefore, TIRFM is the imaging modality of choice to study membrane-delimited proteins with a high signal-to-noise ratio in fixed and live samples.
Materials and Reagents
Lens cleaning tissue (Thor Labs, catalog number: MC-50E)
#1.5 coverslip (Warner Instruments, catalog number: CS-15R)
35 mm tissue culture-treated dishes (Fisherbrand, Fisher Scientific, catalog number: FB012920)
60 mm tissue culture-treated dishes (Fisherbrand, Fisher Scientific, catalog number: FB012921)
Immersion oil (Cargille Laboratories, catalog number: 16241)
Dulbecco’s modified Eagle medium (ATCC, catalog number: 30-2002)
OptiMEM (Gibco, catalog number: 02634)
0.05% trypsin (Cytiva, catalog number: SH30236.01)
Polyethylenimine (PEI) (Polysciences, catalog number: 24765-1)
Human embryonic kidney 293-T cells (ATCC, catalog number: CRL-3216)
Fetal bovine serum (R&D Systems, catalog number: S12450)
Penicillin/streptomycin (Cytiva, catalog number: SV30010)
CIBN-CAAX (Dr. Pietro De Camilli, Addgene ID# 79574)
mCherry-CRY2-5PtaseOCRL (Dr. Pietro De Camilli, Addgene ID# 66836)
mCherry-CRY2–mPKCϵ-CAT-HA (Gada et al., 2022, Addgene ID# 190483)
NaCl (Fisher, catalog number: S271)
KCl (Fisher, catalog number: P217)
MgCl2 (Fisher, catalog number: M33)
CaCl2 (Fisher, catalog number: C79)
HEPES (Oakwood Chemical, catalog number: 047861)
TIRF imaging solution (see Recipes)
Equipment
Lasers (Coherent, OBIS LX445 1185051, OBIS LS 561 1253301)
Camera (Teledyne Photometrics, Prime95B)
Stage (Mad City Labs, X-Y-Z nanopositioning, model: Nano-LPS)
Any in-house or commercial microscope frame that can be modified to hold micromirrors below the objective lens. We use an RM21 frame from Mad City Labs Inc.
Micromirrors are available from Mad City Labs
High numerical aperture (NA) objective lens (e.g., Olympus 60×, 1.5 NA from Olympus)
Quick release magnetic sample holder (Warner Instruments, model: QR-42LP)
Software
Micromanager (University of California at San Francisco, https://micro-manager.org)
ImageJ (National Institute of Health/Wayne Rasband, https://imagej.nih.gov/ij/). Alternatively, use FIJI (ImageJ) software; a build of ImageJ with several prepopulated Plugins and Macros for data analysis.
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gada, K. D., Kamuene, J. M., Kawano, T. and Plant, L. D. (2023). Imaging Membrane Proteins Using Total Internal Reflection Fluorescence Microscopy (TIRFM) in Mammalian Cells. Bio-protocol 13(4): e4614. DOI: 10.21769/BioProtoc.4614.
- Gada, K. D., Kawano, T., Plant, L. D. and Logothetis, D. E. (2022). An optogenetic tool to recruit individual PKC isozymes to the cell surface and promote specific phosphorylation of membrane proteins. J Biol Chem 298(5): 101893.
分类
生物物理学 > 显微技术
细胞生物学 > 细胞信号传导 > 磷酸化
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link