- EN - English
- CN - 中文
A CRISPR-based Strategy for Temporally Controlled Site-Specific Editing of RNA Modifications
基于 CRISPR 的 RNA 修饰的时间控制位点特异性编辑策略
(*contributed equally to this work) 发布: 2023年02月05日第13卷第3期 DOI: 10.21769/BioProtoc.4607 浏览次数: 1864
评审: David PaulKenji SugiyamaAnonymous reviewer(s)

相关实验方案
利用EpiCRISPR系统通过靶向DNA甲基化诱导Alpha TC1-6细胞产生胰岛素
Marija B. Đorđević [...] Melita S. Vidaković
2025年10月20日 1230 阅读
Abstract
Chemical modifications on RNA play important roles in regulating its fate and various biological activities. However, the impact of RNA modifications varies depending on their locations on different transcripts and cells/tissues contexts; available tools to dissect context-specific RNA modifications are still limited. Herein, we report the detailed protocol for using a chemically inducible and reversible platform to achieve site-specific editing of the chosen RNA modification in a temporally controlled manner by integrating the clustered regularly interspaced short palindromic repeats (CRISPR) technology and the abscisic acid (ABA)-based chemically induced proximity (CIP) system. The procedures were demonstrated using the example of inducible and reversible N6-methyladenosine (m6A) editing and the evaluation of its impact on RNA properties with ABA addition and reversal with the control of ABA or light.
Keywords: CRISPR (CRISPR)Background
More than 170 chemical modifications on RNA have been discovered, which contribute to the regulation of genetic information (Licht and Jantsch, 2016; Ontiveros et al., 2019; Boccaletto et al., 2022). Among all RNA modifications, some are known to be dynamic and reversible, fine-tuned by several endogenous writers, erasers, and reader proteins in cells, which are responsible to generate, remove, and interpret these RNA modifications, respectively (Wang and He, 2014; Xu and Liang, 2022). The most prevalent dynamic RNA modification, N6-methyladenosine (m6A), is deposited by methyltransferase-like 3 (METTL3) and other auxiliary proteins, such as methyltransferase-like 14 (METTL14) and Wilms’ tumor 1associated protein (WTAP) (Wang and He, 2014; Yang et al., 2018). The m6A erasers consist of the fat mass and obesity–associated protein and ALKB homologue 5 (ALKBH5), while heterogenous nuclear ribonucleoproteins and the YTH protein family recognize m6A and facilitate its function (N. Liu and Pan, 2016; Yang et al., 2018). With the rapid advance in the field of RNA epigenetics, consensus has been reached that RNA modifications play important roles in regulating its fate and orchestrating various associated biological processes. Nevertheless, the relationships between a particular RNA modification in specific contexts and the observed phenotypes are still elusive, due to limitations in the current strategies to dissect the functions of RNA modifications in a context-dependent and temporally controlled manner. Strategies to investigate RNA modifications relying on the genetic overexpression and knockdown or knockout of the corresponding enzymes/proteins generate global and irreversible changes, which complicate the functional studies of specific RNA modifications and the understanding of their direct biological consequences.
The last decade has witnessed the flourish of clustered regularly interspaced short palindromic repeats (CRIPSR), which can be reprogrammed for various biological and biomedical applications (Pickar-Oliver and Gersbach, 2019;Lo et al., 2022). There have been different studies using the nuclease inactive Cas (dCas) protein to recruit m6A writers, erasers, and readers to specific sites on RNA that are accessible for m6A editing (Rauch et al., 2018; X. M. Liu et al., 2019; Wilson et al., 2020). These methods achieved site-specific m6A editing, taking the advantage of the targeting ability from the combination of dCas protein and single guide RNA (sgRNA). Unfortunately, the temporal control in these RNA modifications editing processes remains lacking.
To address this critical issue, we recently reported a new strategy by integrating CRISPR with the abscisic acid (ABA)-based chemically induced proximity (CIP) system (Shi et al., 2022). The CRISPR-CIP integrated methods have been used to provide inducible and reversible control of epigenome editing (Chen et al., 2017) and site-specific m6A modification editing (Shi et al., 2022), under the regulation of small molecule ABA. The ABA-controlled inducible platform for m6A writing was designed via individually fusing the dCas13b protein and the m6A writer, METTL3, with PYL and ABI (two inducer-binding domains of ABA). The easy reprogramming nature of this platform makes it a promising tool to target various RNAs of interest by switching the target sequences of sgRNA to achieve site-specific m6A writing. In addition, the m6A writers can be replaced by erasers to achieve site-specific m6A removal, which implies a great potential for achieving editing of other RNA modifications by employing corresponding enzymes. We expect that this versatile inducible tool can be adapted for editing other RNA modifications, which opens a new door for targeted RNA modification editing in a temporally controlled manner.
In this protocol, we describe the experimental details for developing and evaluating the inducible and reversible m6A modification editing platform (Shi et al., 2022). The design of this platform is in the scheme below (Figure 1).
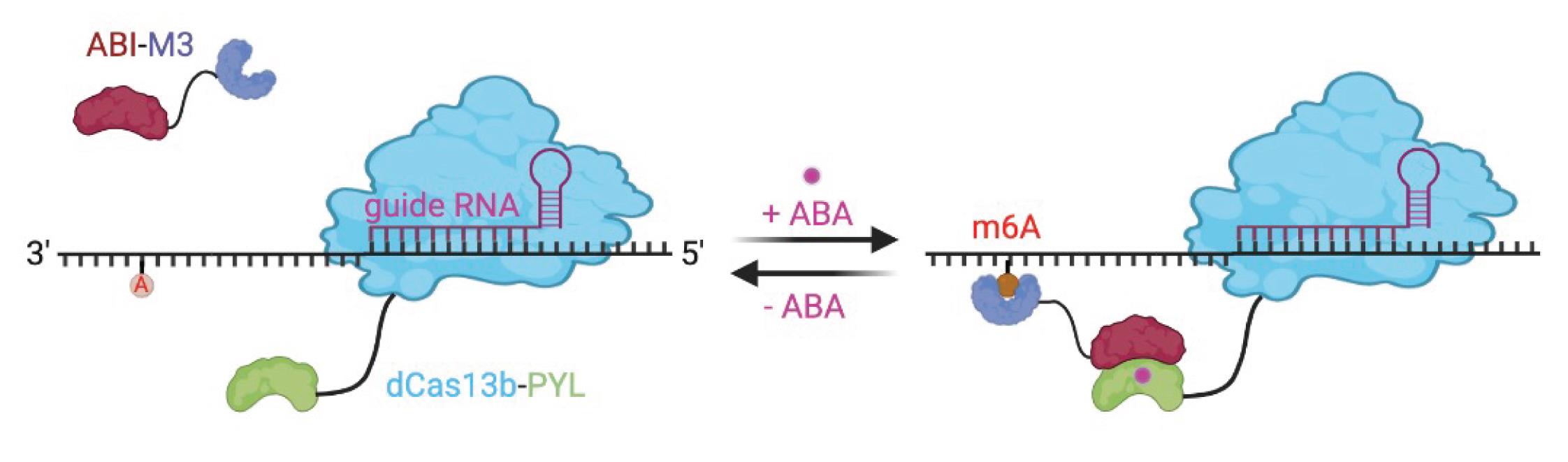
Figure 1. Reversible and inducible m6A editing platform. The targeted m6A editing is triggered by the addition of ABA, which can be reversed by ABA removal. The figure was created with Biorender.com.
Materials and Reagents
Universal pipet tips, 10 µL (VWR, catalog number: 76323-396)
Universal pipet tips, 200 µL (VWR, catalog number: 76323-390)
Universal pipet tips, 1,250 µL (VWR, catalog number: 76323-456)
Microcentrifuge tubes (VWR, catalog number: 87003-294)
1.7 mL culture tubes (VWR, catalog number: 60818-689)
50 mL centrifuge tube (Celltreat, catalog number: 229421)
BioDot pure8-strip PCR tubes (Dot Scientific, catalog number: 415-8PCR)
100 mm TC-treated cell culture dish (Falcon, catalog number: 353003)
Tissue culture plates, 6 well, sterile (VWR, catalog number: 10062-892)
Tissue culture plates, 12 well, sterile (VWR, catalog number: 10062-894)
Tissue culture plates, 24 well, sterile (VWR, catalog number: 10062-896)
500 mL solution bottle, sterile (Celltreat, catalog number: 229784)
500 mL bottle top filter, sterile (Celltreat, catalog number: 229717)
Universal pipet tips, 10 µL, filtered, pre-sterile (VWR, catalog number: 76322-528)
Universal pipet tips, 20 µL, filtered, pre-sterile (VWR, catalog number: 76322-134)
Universal pipet tips, 200 µL, filtered, pre-sterile (VWR, catalog number: 76322-150)
Universal pipet tips, 1,250 µL, filtered, pre-sterile (VWR, catalog number: 76322-528)
Disposable serological pipets, 2 mL (VWR, catalog number: 75816-104)
Disposable serological pipets, 5 mL (VWR, catalog number: 89130-896)
Disposable serological pipets, 10 mL (VWR, catalog number: 89130-898)
Disposable serological pipets, 25 mL (VWR, catalog number: 89130-900)
HEK293T cell (gift from Dr. Gerald R Crabtree at Stanford University Medical School)
HeLa cell (gift from Dr. Gerald R Crabtree at Stanford University Medical School)
Q5 High-Fidelity 2× Master Mix (New England BioLabs, catalog number: M0492S)
Monarch® PCR & DNA Cleanup kit (New England Biolabs, catalog number: T1030S)
NotI-HF restriction enzyme (New England BioLabs, catalog number: R3189)
EcoRI-HF restriction enzyme (New England BioLabs, catalog number: R3101S)
PmeI restriction enzyme (New England BioLabs, catalog number: R0560S)
BbsI restriction enzyme (New England BioLabs, catalog number: R0539S)
HindIII-HF restriction enzyme (New England BioLabs, catalog number: R3104S)
KpnI-HF (New England BioLabs, catalog number: R3142)
CutSmart buffer (New England BioLabs, catalog number: B7204)
Bst 2.0 DNA polymerases (New England BioLabs, catalog number: M0537S)
SplintR ligase (New England BioLabs, catalog number: M0375S)
ATP solution (100 mM) (Thermo Scientific, catalog number: R0441)
pCMV-dCas13-M3nls (Addgene, catalog number: 155366)
pC0043-PspCas13b-crRNA (Addgene, catalog number: 103854)
QIAquick Gel Extraction kit (QIAGEN, catalog number: 28704)
Agarose I (Thermo Fisher Scientific, catalog number: 17852)
T4 DNA ligase reaction buffer (New England BioLabs, catalog number: B0202A)
T4 DNA ligase (New England Biolabs, catalog number: M0202L)
In-Fusion Snap Assembly bundles for seamless DNA cloning kit (Takara, catalog number: 638952)
1 kb plus DNA ladder (New England BioLabs, catalog number: N3200)
T4 polynucleotide kinase (New England BioLabs, catalog number:M0201S)
TAE buffer (Tris-acetate-EDTA) (50×) (Thermo Fisher Scientific, catalog number: B49)
Ampicillin sodium salt (Sigma, catalog number: A9518)
Nuclease-free water (Sigma, catalog number: BCCG1748)
Dulbecco’s modified Eagle’s medium, high glucose (Sigma, catalog number: RNBK9791)
Opti-MEM reduced serum medium (OMEM), GlutaMax supplement (Gibco, catalog number: 51985034)
Fetal bovine serum, premium selected (R&D Systems, catalog number: S11550)
Lipofectamine 2000 transfection reagent (Invitrogen, catalog number: 11668019)
TRIzol reagent (Invitrogen, catalog number: 15596026)
Choloroform:isoamyl alcohol 24:1 (Sigma, catalog number: MKCM1807)
2-propanol (Sigma, catalog number: 19516)
Ethanol (Fisher Bioreagents, catalog number: BP28184)
RIPA buffer (Thermo Fisher Scientific, catalog number: 89901)
Protease inhibitor cocktail (Thermo Fisher Scientific, catalog number: 78430)
Dulbecco’s phosphate buffered saline (PBS) (Sigma, catalog number: D8537)
PierceTM 660 nm protein assay reagent (Thermo Fisher Scientific, catalog number: 22660)
2-mercaptoethanol (Sigma, catalog number: M6250)
4× Laemmli sample buffer (Bio-Rad, catalog number: 1610747)
EZ-Run prestained protein (Fisher Bioreagents, catalog number: BP36011)
4%–15% precast polyacrylamide gel (Bio-Rad, catalog number: 4561084)
Methanol (Fisher Scientific, catalog number: A412-4)
Non-fat milk (Bio-Rad, catalog number: 1705016)
Tween-20 Surfact-AmpsTM detergent solution (Thermo Scientific, catalog number: PI85113)
Anti-HA Tag monoclonal antibody (2-2.2.14) (Invitrogen, catalog number: 26183)
Anti-Flag Tag monoclonal antibody (M2) (Sigma, catalog number: F1804)
Anti-Gapdh antibody GAPDH (D16H11) XP® Rabbit mAb (Cell Signaling Technology, catalog number: 5174)
Anti-FOXM1(A-11) (Santa Cruz, catalog number: sc-271746)
Anti-SOX2 (E-4) (Santa Cruz, catalog number: sc-365823)
Anti-vinculin (E1E9V, 1:1,000) (Cell Signaling Technology, catalog number: 13901)
Anti-rabbit IgG HRP-linked antibody (Cell Signaling Technology, catalog number: 7074)
Anti-mouse IgG HRP-linked antibody (Cell Signaling Technology, catalog number: 7076)
ClarityTM Western ECL substrate (Bio-Rad, catalog number: 1705060)
Immuno-Blot PVDF membrane (Bio-Rad, catalog number: 1620177)
4% paraformaldehyde (Thermo Fisher Scientific, catalog number: J19943.K2)
TritonTM X-100 surfact-AmpTM detergent solution (Thermo Fisher Scientific, catalog number: 85111)
Alexa Fluor Plus 488 (Thermo Fisher Scientific, catalog number: 32723)
DAPI (Fisher Scientific, catalog number: NBP2311561)
Actinomycin D (Thermo Fisher Scientific, catalog number: 11805017)
RNA clean & concentrator-5 (Zymo Research, catalog number: R1014)
N6-Methyladenosine (m6A) (D9D9W) rabbit mAb (Cell Signaling Technology, catalog number: 56593)
PierceTM Protein G magnetic beads (Thermo Fisher Scientific, catalog number: 88848)
N6-Methyladenosine 5′-monophosphate sodium salt (m6A salt) (Sigma, catalog number: M2780-10MG)
Protease K solution, RNA grade (Invitrogen, catalog number: 25530049)
RNasin Plus ribonuclease inhibitor (Promega, catalog number: N2611)
Ultrapure bovine serum albumin (BSA), 50 mg/mL (Invitrogen, catalog number: AM2616)
Zinc chloride (ZnCl2) (Sigma, catalog number: Z0152)
0.5 M EDTA pH 8.0 (Invitrogen, catalog number: AM9260G)
Formaldehyde solution (Sigma, catalog number: F8775-500ML)
Anti-METTL3 antibody (Invitrogen, catalog number: 15073-1-AP)
Glycine (Fisher bioreagents, catalog number: BP181-1)
Magnesium chloride solution, 1 M (MgCl2) (Sigma, catalog number: M1028-100ML)
Sodium dodecyl sulfate (SDS) (Sigma, catalog number: L3771-500G)
5 M sodium chloride (NaCl) (Invitrogen, catalog number: AM9759)
1 M Tris-HCl, pH 7.0 (Sigma, catalog number: T1819-1L)
1 M Tris-HCl, pH 7.4 (Sigma, catalog number: T2663-1L)
IGEPAL-CA-630 (Sigma, catalog number: I8896)
Sodium acetate (3 M), pH 5.5, RNase-free (Invitrogen, catalog number: AM9740)
Pierce RIPA buffer (Thermo Fisher Scientific, catalog number: 89900)
Halt protease inhibitor cocktail (Thermo Scientific, catalog number: 1861279)
PowerUpTM SYBRTM Green Master Mix (Thermo Fisher, catalog number: A25778)
iScript cDNA Synthesis kit (Bio-Rad, catalog number: 1708891)
Abscisic acid (ABA) (Gold Biotechnology, catalog number: A-050-5)
Dimethyl sulfoxide (DMSO) (Sigma, catalog number: D8418-100 mL)
Tris base (Sigma, catalog number: T1503-500 g)
Agar (Sigma, catalog number: A6686)
LB media (see Recipes)
LB plate with ampicillin (see Recipes)
1× gel running buffer (see Recipes)
1× membrane transfer buffer (see Recipes)
1× TBST buffer (see Recipes)
Fragmentation buffer, 10× (see Recipes)
5× IP buffer for m6A-IP study (see Recipes)
m6A elution buffer (see Recipes)
Wash buffer for RNA-crosslink immunoprecipitation (see Recipes)
NT-2 buffer, 5× (see Recipes)
Protease K digestion buffer (see Recipes)
Equipment
Centrifuge 5424/5424R (Eppendorf, catalog number: EP5404000537)
New Brunswick I26/I26R stackable incubator shakers (Eppendorf, catalog number: VWR-89173-938)
Sorvall LYNX 6000 superspeed centrifuge (Thermo Scientific, catalog number: 75006590)
7900HT Fast Real-Time PCR system (Applied Biosystems, catalog number: 4329001)
ChemiDoc MP imaging system (Bio-Rad, catalog number: 17001402)
C1000 touch thermal cycler (Bio-Rad, catalog number: 1851148)
NanoDrop One spectrophotometer (Thermo Scientific, catalog number: ND-ONE-W4)
Bioruptor Pico sonication device (Diagenode, catalog number: B01060010)
Fluorescence microscopy (Agilent BioTek LFXSN, catalog number: BTFLX)
CriterionTM Blotter with wire electrodes (Bio-Rad, catalog number: 1704071)
Procedure
文章信息
版权信息
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Xu, Y., Wang, Y. and Liang, F. S. (2023). A CRISPR-based Strategy for Temporally Controlled Site-Specific Editing of RNA Modifications. Bio-protocol 13(3): e4607. DOI: 10.21769/BioProtoc.4607.
分类
细胞生物学 > 细胞工程 > CRISPR-cas9
分子生物学 > RNA > 表观转录组
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link