- EN - English
- CN - 中文
Establishment of Restraint Stress–induced Anorexia and Social Isolation–induced Anorexia Mouse Models
束缚应激诱导的厌食症和社会隔离诱导的厌食症小鼠模型的建立
发布: 2023年01月20日第13卷第2期 DOI: 10.21769/BioProtoc.4597 浏览次数: 1835
评审: Edgar Soria-GomezAbraam YakoubMiao He
Abstract
Anorexia nervosa (AN) is a devastating neuropsychiatric disease with a prevalence rate of approximately 0.3%–1% among women and morbidity and mortality rates among the highest of all neuropsychiatric disorders. The disease etiology is complex but primarily characterized by reduced food intake and body weight, and intense anxiety and fear associated with gaining weight. Existing rodent models of AN are useful and capture features of the disease, but either require specialized genetic mouse models or are difficult to implement in mice. Here, we describe two simple mouse models of stress-induced anorexia that are easy to implement in basic research labs, and capture core features associated with AN, such as reduced food intake in the context of social/physical stress and increased anxiety-related behavior. These protocols provide reproducible and robust assays for stress-induced anorexia and may be implemented with additional assays to probe the neural circuitry mediating the effects of psychological stress on feeding in mice.
Graphical abstract
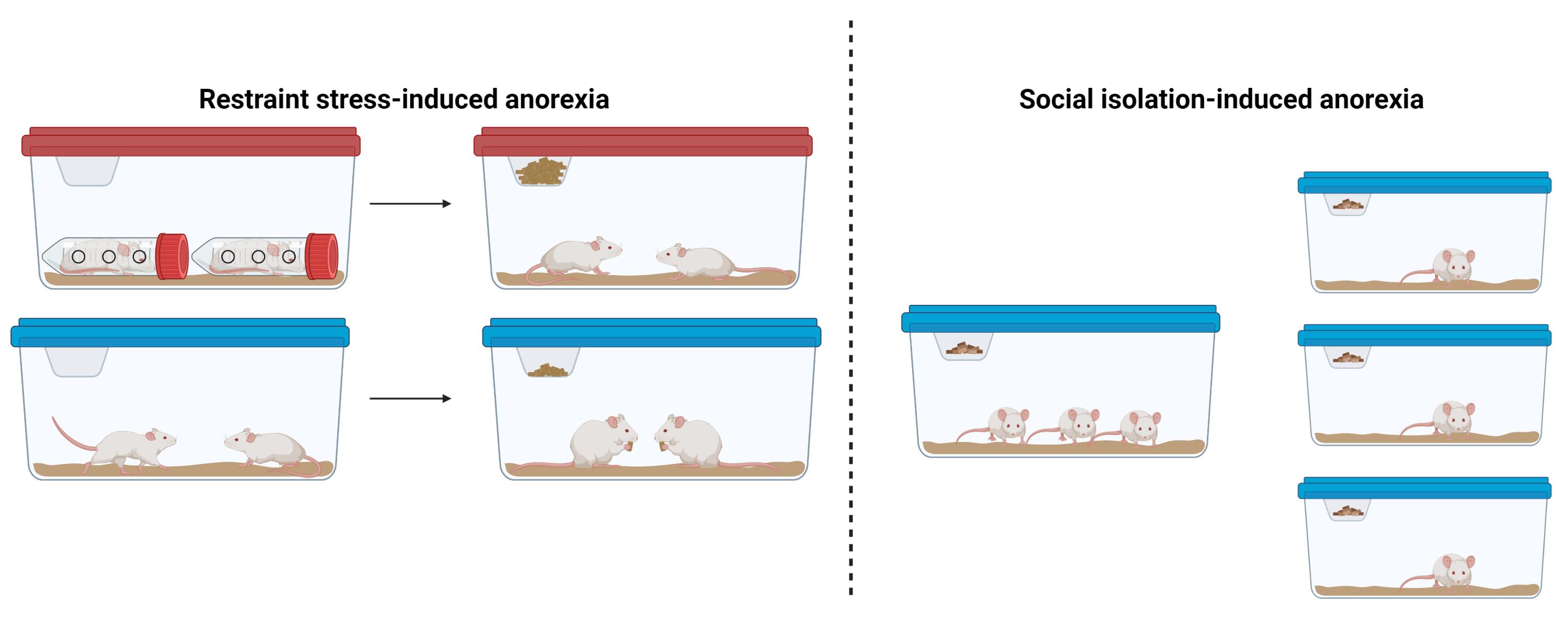
Background
Feeding behavior is controlled by multiplex neural circuitry throughout the brain, including the hypothalamus, hindbrain, and mesolimbic dopamine circuitry (Sohn et al., 2013). While dedicated feeding circuits exist in the brain, substantial experimental evidence indicates that these are bidirectionally connected to neural circuitry controlling stress and emotion (Sweeney and Yang, 2017). These interactions are especially important when considering the biological mechanisms governing neuropsychiatric eating disorders such as anorexia nervosa (AN), since eating disorders are characterized by alterations in both feeding and emotional state (Keski-Rahkonen, 2007). As such, the underlying biological causes of eating disorders may be mediated by neural circuit alterations in brain circuits at the intersection of feeding and emotion. Thus, mouse models of stress-induced anorexia have value in dissecting the neural circuit and molecular mechanisms for normal and pathological feeding responses to stress.
Anorexia nervosa is a serious neuropsychiatric disorder characterized by reduced feeding and body weight and co-morbid neuropsychiatric traits, such as increased anxiety and depressive-related behaviors (Bulik et al., 2006). The disease occurs in approximately 0.3%–1% of women in the United States and is associated with a high rate of morbidity and mortality (Bulik et al., 2006). Since the disease is characterized by reduced feeding in the context of psychological and psychosocial stress, substantial efforts have been made to model aspects of AN in rodents (Scharner and Stengel, 2020; Zhang and Dulawa, 2021). Although AN is a complex disease with human-specific etiology, important components of the disease can be effectively modeled in rodents, such as reduced feeding in the face of stress and anxiety, compulsive exercise, and elevated anxiety and depressive behavior. These models provide an important entry point towards understanding the biological mechanisms mediating dysregulated eating and may provide a pathway towards novel therapeutic options for eating disorders.
One commonly used rodent model of AN is the activity-based anorexia (ABA) mouse model (Carrera et al., 2014). The ABA model subjects rodents (mice or rats) to restricted food access in the presence of a running wheel. In this model, a subset of mice will stop eating and exercise excessively, leading to starvation and death. This model is useful as it incorporates multiple characteristics of AN, such as excessive exercise and voluntary anorexia, and is more effective in female rodents (approximately 90% of AN cases occur in females). However, the model relays on the presence of a running wheel and restricting food access to a user-defined time window (usually between 1 and 4 h/day). Rodents in this model develop gradual hypothermia and may exercise excessively to generate heat. As such, the ABA model does not work when supplemental heat is provided to the rodents (Gutierrez et al., 2008). Thus, although hypothermia is often observed in AN, the ABA model may more closely model physiological forms of anorexia resulting secondarily to hypothermia. Further, the model can be difficult to implement in mice (as opposed to rats), and only a subset of mice are sensitive to the ABA model.
Given the limitations of the ABA model, additional attempts have been made to develop mouse models that capture the AN phenotype more accurately. One model that shows promise is the “GED” (gene, environment, deprivation) model developed in the lab of Lori Zeltser (Madra and Zeltser, 2016; Madra et al., 2015). The GED model uses a transgenic mouse line containing a BDNF-Val66Met mouse mutation that has been associated with neuropsychiatric disorders. In the GED model, the BDNF-Val66Met mice are subjected to social isolation during adolescence (environmental manipulation) and given 20%–30% calorically restricted food access during a user-defined time window of 3 h twice a day. Thus, this model incorporates a genetic component (BDNF-Val66Met mutation), an environmental stressor (social isolation), and food deprivation by both caloric restriction and limited food access to produce anorexia-related behaviors. A subset of mice exposed to the GED model voluntarily stop eating and develop severe anorexia. Like in the ABA model, in the GED model the voluntary starvation phenotype observed in AN is captured and the starvation phenotype is more common in female mice. However, this model relays on a transgenic mouse line, which limits the use of some modern behavioral neuroscience approaches, such as optogenetics and chemogenetics, which often require additional transgenic mouse lines and/or multiple breeding steps. Therefore, there is a need for simplified stress-induced anorexia models which can be implemented in all basic biology labs and transgenic mouse lines (Table 1).
Here, we describe two simple models of stress-induced anorexia that are easy to implement in mice: restraint stress–induced anorexia and social isolation–induced anorexia. These models do not require specialized equipment or transgenic mouse strains and can be implemented in most biology and neuroscience laboratories. Both of these assays may serve as useful models for disorders characterized by reduced feeding in the face of physical or psychosocial stress, such as the reduced appetite associated with stress-related mood disorders or the impaired food-seeking and consumption observed in AN.
Table 1. Strengths and weaknesses of common models of stress-induced anorexia
| Method | Advantages | Disadvantages |
|---|---|---|
| Activity-based anorexia | -Easy to implement -Self-induced anorexia and starvation -Excessive exercise -More severe in female rodents | -Model may rely on physiological anorexia secondary to hypothermia, as opposed to psychologically induced anorexia -Difficult to implement in mice (relative to rats) |
| G.E.D. model | -More severe in females -Self-induced anorexia and starvation in a subset of mice -Anorexia due to a combination of genetic and environmental factors | -Requires a specialized transgenic mouse line |
| Restraint induced–anorexia | -Easy to implement -Reliably induced anorexia and weight loss in mice -Easily combined with advanced behavioral neuroscience approaches (i.e., optogenetics, chemogenetics) | -Not sexually dimorphic -Usually does not induce self-starvation -May not capture psychologically induced anorexia |
| Social isolation–induced anorexia | -Easy to implement -Anorexia resulting from chronic psychological stress | -Anorexia phenotype is usually mild -Better suited for determining factors that enhance psychological-induced anorexia -Unknown sexual dimorphism |
Subjects and housing: Adult male and female C57/BL6J littermate mice may be used for both social isolation and restraint stress–induced anorexia studies. Given that neuropsychiatric eating disorders are highly sexually dimorphic, it is important to include both sexes in the experimental design and to determine if behavioral phenotypes are differentially expressed in males and females. For restraint stress–induced anorexia assays, littermate mice should be group-housed with 3–4 mice per cage. Lights in the housing facility should be on a 12:12 h light/dark cycle. Since mice are nocturnal and consume most of their food during the dark period, it is recommended to perform feeding assays at the start of the dark cycle, although light cycle measurements may also be performed.
Materials and Reagents
Group-housed mice
50 mL conical tubes with holes cut into the sides (for restraint stress–induced anorexia; Figure 1)
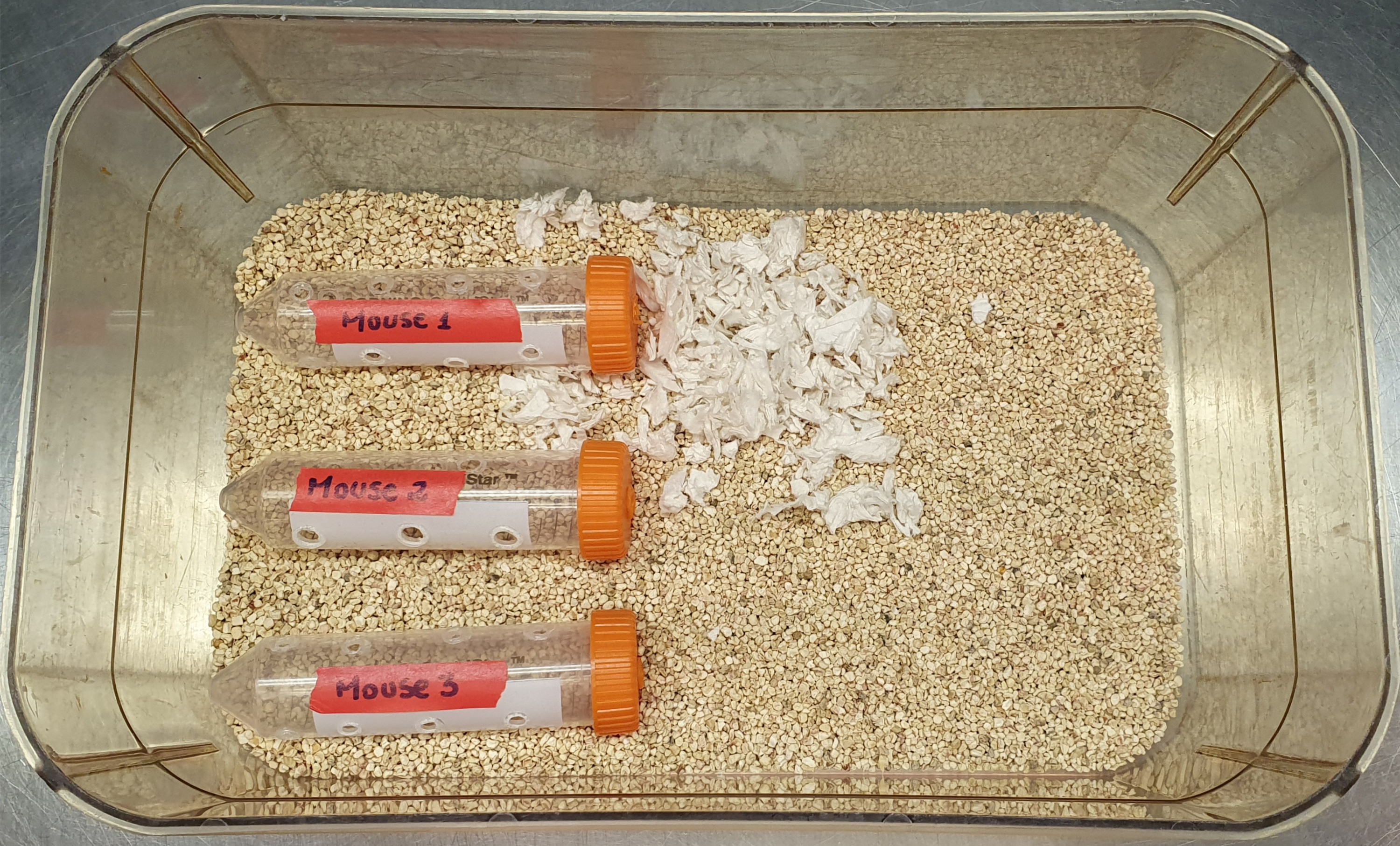
Figure 1. Setup of restraint stress–induced anorexia apparatus. Separate cages are used for male and female mice, respectivelyClean mouse cages
Standard chow mouse diet (Formulab Diet 5008 or similar approved mouse diet)
Standard home cage water bottles
Standard food hoper
Cage enrichment (such as nestlets)
Scale for measuring food intake and body weight (Satorius Entris II Essential Precision Balance or similar)
Lab notebook for recording food intake and body weight
Instructions to make restraint stress apparatus
A simple restraint stress–anorexia apparatus can be constructed from 50 mL conical tubes (Figure 1). Make 6–12 holes, depending on how many you can fit, on the sides of the conical tubes to enable mice to breathe. Care should be taken to make the holes big enough for air flow, but not big enough for the mice to fit their nose into, so they do not hurt themselves in the borders. Holes can be made by using a tool such as a heat pen for melting plastic. The mouse identifying information (i.e., ear tag number and sex) can be written on the side of each tube. During restraint sessions, the mice can be placed into the restrainers within their resident cage (Figure 1). Following each restraint session, the restraint tubes should be cleaned with soap and water and allowed to dry before re-using on each mouse.
Procedure
文章信息
版权信息
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Possa-Paranhos, I. C., Catalbas, K., Butts, J., O’Berry, K. and Sweeney, P. (2023). Establishment of Restraint Stress–induced Anorexia and Social Isolation–induced Anorexia Mouse Models. Bio-protocol 13(2): e4597. DOI: 10.21769/BioProtoc.4597.
- Sweeney, P., Bedenbaugh, M. N., Maldonado, J., Pan, P., Fowler, K., Williams, S. Y., Gimenez, L. E., Ghamari-Langroudi, M., Downing, G., Gui, Y., et al. (2021). The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci Transl Med 13(590): eabd6434.
分类
神经科学 > 行为神经科学
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









