- EN - English
- CN - 中文
PERK Pathway Inhibitors Cure Group A Streptococcal Necrotizing Fasciitis in a Murine Model
PERK 通路抑制剂可治愈小鼠模型中的 A 组链球菌坏死性筋膜炎
(*contributed equally to this work) 发布: 2022年12月20日第12卷第24期 DOI: 10.21769/BioProtoc.4589 浏览次数: 2122
评审: Luis Alberto Sánchez VargasKarolina SubrtovaAnonymous reviewer(s)
Abstract
Group A streptococcus (GAS) is a Gram-positive human pathogen that causes invasive infections with mild to life-threatening severity, like toxic shock syndrome, rheumatic heart disease, and necrotizing fasciitis (NF). NF is characterized by a clinical presentation of widespread tissue destruction due to the rapid spread of GAS infection into fascial planes. Despite quick medical interventions, mortality from NF is high. The early onset of the disease is difficult to diagnose because of non-specific clinical symptoms. Moreover, the unavailability of an effective vaccine against GAS warrants a genuine need for alternative treatments against GAS NF. One endoplasmic reticulum stress signaling pathway (PERK pathway) gets triggered in the host upon GAS infection. Bacteria utilize asparagine release as an output of this pathway for its pathogenesis. We reported that the combination of sub-cutaneous (SC) and intraperitoneal (IP) administration of PERK pathway inhibitors (GSK2656157 and ISRIB) cures local as well as systemic GAS infection in a NF murine model, by reducing asparagine release at the infection site. This protocol's methodology is detailed below.
Background
Group A Streptococcus (GAS), or Streptococcus pyogenes , is a human-specific Gram-positive bacterial pathogen. It remains among the top ten pathogens causing several diseases with mild to life-threatening clinical symptoms (Bisno and Stevens, 1996;Efstratiou and Lamagni, 2016). The clinical presentation of these diseases includes pharyngitis, impetigo, erysipelas, tonsilitis, scarlet fever, necrotizing fasciitis, rheumatic fever, glomerulonephritis, septicemia, and others. More than 220 different GAS serotypes are circulating in the human population worldwide. M1T1 is the dominant serotype spread globally and causing diseases in the population of economically well-equipped countries (Aziz and Kotb, 2008; Nelson et al., 2016; Lynskey et al., 2019). GAS classification is based on variation in the N-terminal amino acid sequence of the surface M-protein. Antibiotic regimens are the only definitive strategy to cure GAS infections, because of the unavailability of any licensed universal vaccine on the market. Recent reports on the development of antibiotic resistance in GAS are worrisome and have motivated us to develop a new alternative therapeutic approach to cure its infections.
Invasive GAS infections, like sepsis, bacteremic pneumonia, and necrotizing fasciitis (NF), spread in sterile sites (Metzgar and Zampolli, 2011). NF begins as a small lesion with the appearance of mild erythema, which rapidly progresses with inflammation in the subcutaneous tissue, and destructs skin and soft tissue over large body areas (Walker et al., 2014). Antibiotic administration, surgical debridement of infected tissues, and supportive care are the main line of treatment for invasive GAS diseases (Stevens and Bryant, 2017). Unfortunately, the mortality rate from NF ranges from 23 to 35% in resource-rich settings, despite quick medical interventions (Davies et al., 1996; Carapetis et al., 2005;Allen and Moore, 2010;Olsen and Musser, 2010; Cole et al., 2011;Ralph and Carapetis, 2013). Therefore, alternative therapeutic approaches are urgently needed to combat invasive soft-tissue GAS infections.
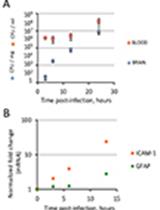
Our recent studies showed that GAS causes endoplasmic reticulum stress (ER stress) and triggers the unfolded protein response (UPR) in the host cells upon infection. This results in enhanced asparagine synthesis and release, which is utilized by GAS to increase virulence and rate of proliferation. Furthermore, the PERK-eIF2α-ATF4 branch of UPR was exclusively triggered during GAS infection (Anand et al., 2021). When we treated GAS-infected mice with PERK pathway inhibitors (GSK2656157 and ISRIB), the bacteria were cleared faster than in untreated infected mice. We discuss the method of treatment using PERK pathway inhibitors in a murine model of human soft tissue infection.
Materials and reagents
Preparation of bacteria for mice infection
15 mL conical tubes (Greiner Bio-One CELLSTAR, catalog number: 188621)
50 mL conical tubes (MiniPlast, catalog number: 835-050-21-111)
Microcentrifuge tubes (Corning® Axygen, catalog number: MCT-175-C)
Spectrophotometric cuvettes (Polystyrene cuvette, 10 × 4 × 45 mm, Sarstedt AG & Co. KG, Germany, catalog number: 67.742)
GAS strains (serotype M14 strain JS14, which was isolated from a patient with pneumonia, and serotype M1T1 strain 5448, a clinical isolate from a patient with necrotizing fasciitis and toxic shock syndrome)
Sterile Dulbecco's Phosphate Buffered Saline (without calcium chloride and magnesium chloride, pH 7.1–7.5, Biological Industries, catalog number: 02-023-1A)
THY media (see Recipes)
Todd-Hewitt broth (Becton, Dickenson, and Company, catalog number: 249240)
Yeast extract (Becton, Dickenson, and Company, catalog number: 212750)
Blood Agar plates (see Recipes)
Defibrinated sheep blood (DSB) (hylabs®, catalog number: PD049)
Mice infection
1 mL syringes without needles (BD PlastipakTM 1 mL Luer, catalog number: 303172)
Needle (30G × 1/2") for syringe (BD MicrolanceTM 3, catalog number: 2025-01)
Bacterial culture prepared for injection (suspended in PBS)
Female BALB/c OlaHsd mice, aged 3–4 weeks, weighing 10–12 g (ENVIGO RMS, Israel Ltd.)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 472301)
Polyethylene glycol (PEG-400) (Sigma-Aldrich, catalog number: 202398)
0.9% sodium chloride saline (Teva Medical, catalog number: AWN1324)
Ketamine-xylazine mix (mixed in 1:6 ratio, respectively, Ketamine-Vetoquinol, Xylazine-20 mg/mL, Eurovet Animal Health B.V.)
Isoflurane (Piramal Critical Care Inc, USA, catalog number: 66794-013-25)
GSK2656157 (Sigma-Aldrich, catalog number: 504651) (see Recipes)
Integrated stress response inhibitor (ISRIB) (Sigma-Aldrich, catalog number: SML0843) (see Recipes)
CFU quantification of GAS in skin biopsy samples
Sterile 2 mL tubes (Abdos Labtech Private Limited, catalog number: P10203)
Sterile Dulbecco's Phosphate Buffered Saline (without calcium chloride and magnesium chloride, pH 7.1–7.5, Biological Industries, catalog number: 02-023-1A)
70% Ethanol (Romaical, 19-19-009102-80)
8.0-mm Punch biopsy (Acu-Punch, Acuderm Inc., catalog number: P825)
Dissection kit—surgical scissors and fine forceps
Blood Agar plates (see Recipes)
Defibrinated sheep blood (DSB) (hylabs®, catalog number: PD049)
Equipment
Spectrophotometer (Amersham Biosciences, Ultraspec 2100 pro, 80-2112-21)
Centrifuge with swing rotor (Sigma, model: 3-16PK)
Vortex mixer (Heidolph reax top, Z655988)
-80 °C freezer (Thermo Scientific, 8926)
37 °C incubator (BINDER GmbH, BD 56)
Digital vernier caliper (Bar Naor Ltd., BN30087-00)
Electronic homogenizer (POLYTRON® 2100 Homogenizers, Kinematica, 05400261)
Electric shaver (Phillips, MG3730/15)
Desiccator jar (SP BEL-ART polycarbonate/polypropylene desiccator, F42032-0000)
Software
GraphPad Prism 5.03 (for statistical analysis and graphical presentation)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Anand, A., Sharma, A., Ravins, M., Johri, A. K., Tirosh, B. and Hanski, E. (2022). PERK Pathway Inhibitors Cure Group A Streptococcal Necrotizing Fasciitis in a Murine Model. Bio-protocol 12(24): e4589. DOI: 10.21769/BioProtoc.4589.
- Anand, A., Sharma, A., Ravins, M., Biswas, D., Ambalavanan, P., Lim, K. X. Z., Tan, R. Y. M., Johri, A. K., Tirosh, B. and Hanski, E. (2021). Unfolded protein response inhibitors cure group A streptococcal necrotizing fasciitis by modulating host asparagine. Sci Transl Med 13(605).
分类
微生物学 > 体内实验模型 > 细菌
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link