- EN - English
- CN - 中文
Cell-derived Matrix Assays to Assess Extracellular Matrix Architecture and Track Cell Movement
评估细胞外基质结构和跟踪细胞运动的细胞衍生基质试验
发布: 2022年12月20日第12卷第24期 DOI: 10.21769/BioProtoc.4570 浏览次数: 3265
评审: Yoshihiro AdachiSalah BoudjadiAnonymous reviewer(s)
Abstract
The extracellular matrix (ECM) is a non-cellular network of macromolecules, which provides cells and tissues with structural support and biomechanical feedback to regulate cellular function, tissue tension, and homeostasis. Even subtle changes to ECM abundance, architecture, and organization can affect downstream biological pathways, thereby influencing normal cell and tissue function and also driving disease conditions. For example, in cancer, the ECM is well known to provide both biophysical and biochemical cues that influence cancer initiation, progression, and metastasis, highlighting the need to better understand cell–ECM interactions in cancer and other ECM-enriched diseases. Initial cell-derived matrix (CDM) models were used as an in vitro system to mimic and assess the physiologically relevant three-dimensional (3D) cell–ECM interactions. Here, we describe an expansion to these initial CDM models generated by fibroblasts to assess the effect of genetic or pharmacological intervention on fibroblast-mediated matrix production and organization. Additionally, we highlight current methodologies to quantify changes in the ultrastructure and isotropy of the resulting ECM and also provide protocols for assessing cancer cell interaction with CDMs. Understanding the nature and influence of these complex and heterogeneous processes can offer insights into the biomechanical and biochemical mechanisms, which drive cancer development and metastasis, and how we can target them to improve cancer outcomes.
Background
The extracellular matrix (ECM) is the non-cellular compartment of all tissues; it is fundamental for cellular processes, providing biomechanical and biochemical cues, which can be sensed by cells activating downstream signaling pathways that can affect cell behavior (Vennin et al., 2017; Cox, 2021; Romani et al., 2021). The ECM is a highly regulated scaffold whose function is dependent on its precise molecular composition and assembly of fibrillar tissue. The highly regulated ECM is an adaptive network of fibers that respond to external forces and tensional strains, whilst also providing cells with biomechanical feedback regulating homeostasis and normal tissue function (Cox, 2021). However, in cancer, the co-option of this tightly organized infrastructure, including ECM deposition and remodeling, can trigger oncogenic signaling, leading to increased cell motility, proliferation, survival, and metastasis (Nicolle et al., 2017; Neesse et al., 2019;Cox, 2021).
Although the cancer stroma was initially thought to predominantly stimulate tumor progression, recently it was shown to also restrain cancer cells (Rhim et al., 2014; Özdemir et al., 2014). Therefore, a fine-tuned approach to target the pro-tumorigenic functions of the stroma while maintaining its anti-tumorigenic roles may provide potential for anti-cancer therapies. Cancer associated fibroblasts (CAFs) are a prominent stromal cell type, which can be activated and educated by cancer cells (Pereira et al., 2019; Sahai et al., 2020). Their ability to synthesize and remodel ECM is a key driver of tumor progression. As such, there is a clear need to study the influence of the ECM on cancer cell behavior and assess the potential of ECM-targeted therapies to alter cellular locomotion and chemosensitivity.
Initial methodologies and protocols to generate cell-derived matrices (CDMs) were designed to better understand the complexity of cell-matrix adhesion and cell surface structures in a 2.5D to 3D context (Cukierman et al., 2001; Cukierman, 2002; Erami et al., 2016; Franco-Barraza et al., 2016). To decipher the multifaceted influence of the ECM on cancer progression and therapeutic response, CDMs can be used to characterize the effect that genetic or pharmacological manipulation have on ECM ultrastructure. Here, we provide a step-by-step protocol for the generation of CDMs and subsequent quantification of matrix abundance, architecture, and organization (Figure 1). This protocol was utilized in our recent publication to assess fine-tuned manipulation of the matrix following focal-adhesion kinase inhibition, to reduce cancer cell locomotion and improve the efficiency of chemotherapy (Murphy et al., 2021). The protocols presented below contain steps that can be modified in a cell type–specific manner, for normal fibroblasts as well as CAFs. We also highlight the utility of CDMs to assess the potential of anti-fibrotic agents to alter ECM production and remodeling, which has recently shown promise to reduce metastasis and improve chemotherapy performance in a range of cancer types (Miller et al., 2015; Rath et al., 2018; Vennin et al., 2017, 2019; Boyle et al., 2020; Wu et al., 2020; Murphy et al., 2021). Furthermore, we provide protocols for assessing cancer cell behavior when seeded on CDMs, which can provide insight into cancer cell proliferation, survival, and single vs. collective cell migration in an ECM-rich environment (Figure 2). Here, CDMs can provide a rapid and pliable readout of stromal and cancer response to genetic and pharmacological intervention in vitro, which can be used to inform subsequent in vivo studies.

Figure 1. Schematic representation of cell-derived matrix (CDM) production and cancer cell seeding on top of CDMs following fibroblast decellularization. Here, fibroblasts are seeded and allowed to grow to confluence (a.) prior to supplementation with ascorbic acid to enhance matrix production (b.). Following matrix production, fibroblasts are decellularized from the matrix (c.) and cancer cells may be seeded on top (d.). As an example timeline (e.), telomerase-immortalized fibroblasts (TIFs) are provided with ascorbic acid 24 h after cell seeding to allow matrix production for six days, prior to decellularization on day 7, followed by matrix analysis or cancer cell seeding and tracking [here shown as the example of pancreatic cancer cells isolated from the KPC ( Kras G12D/+ ; p53 R172H/+ ; Pdx-1Cre ) mouse model of pancreatic cancer (Morton et al., 2010; Murphy et al., 2021)]. Parts a-d are adapted from Murphy et al. (2021), CC BY 4.0 ( https://creativecommons.org/licenses/by/4.0/ ).
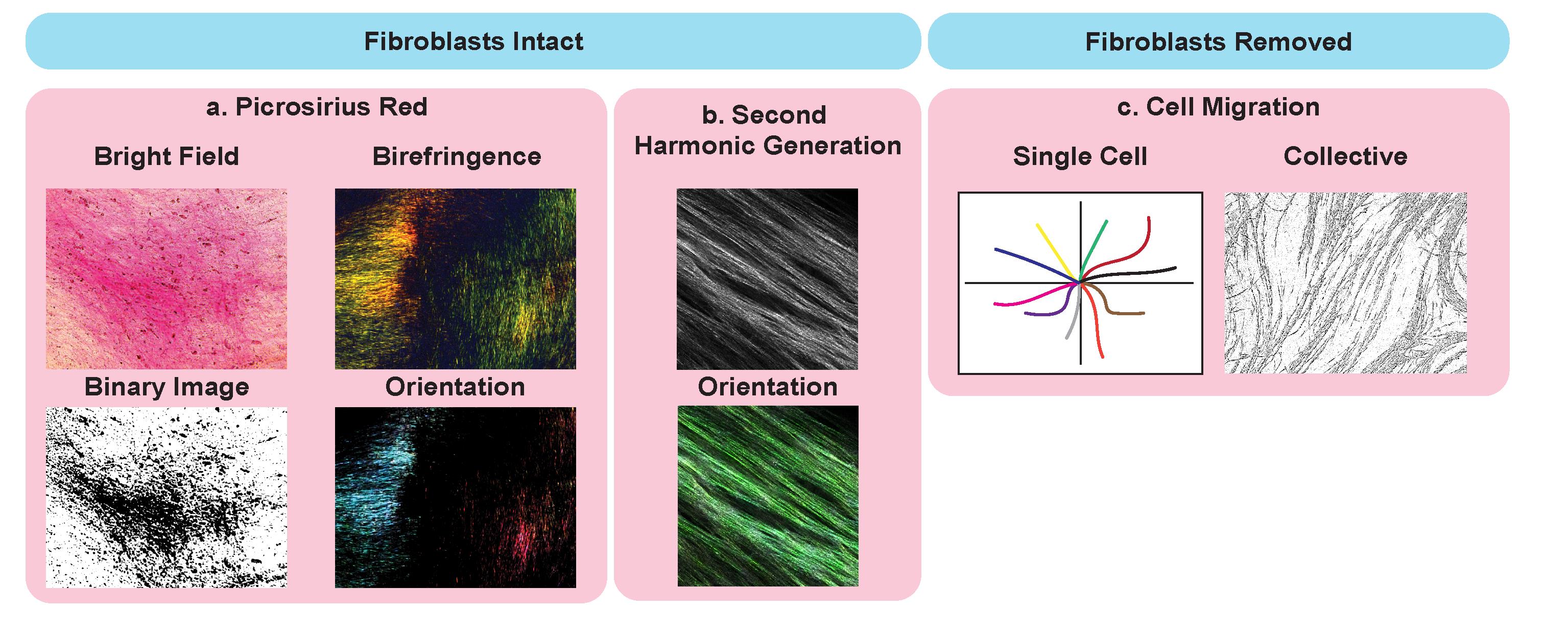
Figure 2. Potential application of cell-derived matrices for analysis of matrix ultrastructure. Examples with intact fibroblasts, including picrosirius red staining (a.) imaged using bright field microscopy (top left), binary overlay (bottom left), and by polarized light (birefringence, top right) to assess collagen I and III abundance and orientation (bottom right). Second-harmonic generation (SHG) (b.) imaging (top) and analysis of fiber orientation (bottom). Applications of CDMs with fibroblast removed and cancer cells seeded on top to assess single cancer cell migration (left) and collective migration (right).
Materials and Reagents
For CDMs
12-well plate
Cultured fibroblasts [e.g., telomerase-immortalized fibroblasts (TIFs) or CAFs]
Trypsin/EDTA solution (Life Technologies, catalog number: 15400)
Fibroblast growth medium [specific to fibroblast line; e.g., for TIFs: DMEM (Thermo Fisher Scientific, catalog number: 11995065) supplemented with 10% FBS]
Phosphate-buffered saline (PBS) (Life Technologies, catalog number: 14190)
Gelatin (Sigma-Aldrich, catalog number: G1393), store at 4 °C
10% neutral buffered formalin (Australian Biostain P/L, catalog number: ANBFC.10L)
Glycine (Sigma-Aldrich, catalog number: G7126)
(+)-Sodium L-ascorbate (Sigma-Aldrich, catalog number: A7631), make fresh
DNase I (Roche, catalog number: 05025), aliquot and store at -20 °C (do not freeze-thaw)
Fungizone (Amphotericin B, Life Technologies, catalog number: 15290)
Penicillin/streptomycin (pen/strep) (Thermo Fisher Scientific, catalog number: 15070-063)
70% ethanol
0.2% sterile gelatin (see Recipes)
1 M sterile glycine (see Recipes)
Ascorbic acid (1,000×), prepare fresh (see Recipes)
Extraction buffer (see Recipes)
Triton X-100 (Sigma-Aldrich, catalog number: 9284)
Ammonium hydroxide
Sodium deoxycholate (Sigma-Aldrich, catalog number: D6750)
Supplemented PBS (see Recipes)
Calcium chloride (Chem Supply, catalog number: CA033)
Magnesium sulfate (Chem Supply, catalog number: MA048)
Supplemented PBS with antibiotics and antifungals (see Recipes)
For picrosirius red staining
0.1% picrosirius red stain (Abcam, catalog number: ab150681)
Phosphomolybdic acid hydrate (Sigma-Aldrich, catalog number: 221856)
Glacial acetic acid (Chem Supply, catalog number: AA009-2.5L-P)
Reverse Osmosis (RO) water
0.02% phosphomolybdic acid (see Recipes)
10% phosphomolybdic acid stock (see Recipes)
Acidified water (see Recipes)
Equipment
12-well glass bottom dishes (Celvis, catalog number: D35-20/4.5N)
Sterile glass bottles (for storage of gelatin, extraction buffer, and glycine)
Picrosirius red staining kit (Australian Biostain, APSRA, 500 mL)
Software
TWOMBLI (Wershof et al., 2021): https://github.com/wershofe/TWOMBLI
FibrilTool (Boudaoud et al., 2014): https://www.quantitative-plant.org/software/fibriltool
Automated ECM fiber orientation and alignment (Mayorca-Guiliani et al., 2017): https://github.com/TCox-Lab/Collagen_Orientation
Automated picrosirius red and collagen birefringence analysis using polarized light (Vennin et al., 2019): https://github.com/TCox-Lab/PicRed_Biref
Cell Tracker (not only) for Dummies (Piccinini et al., 2016): http://celltracker.website/about-celltracker.htmL
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Murphy, K. J., Reed, D. A., Chambers, C. R., Zhu, J., Magenau, A., Pereira, B. A., Timpson, P. and Herrmann, D. (2022). Cell-derived Matrix Assays to Assess Extracellular Matrix Architecture and Track Cell Movement . Bio-protocol 12(24): e4570. DOI: 10.21769/BioProtoc.4570.
- Murphy, K. J., Reed, D. A., Vennin, C., Conway, J. R. W., Nobis, M., Yin, J. X., Chambers, C. R., Pereira, B. A., Lee, V., Filipe, E. C., et al. (2021). Intravital imaging technology guides FAK-mediated priming in pancreatic cancer precision medicine according to Merlin status. Sci Adv 7(40): eabh0363.
分类
癌症生物学 > 微环境
癌症生物学 > 侵袭和转移 > 肿瘤微环境
细胞生物学 > 细胞运动 > 细胞迁移
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link