- EN - English
- CN - 中文
Isolation and Expansion of Primary Conjunctival Stem Cells (CjSCs) from Human and Rabbit Tissues
从人和兔组织中分离和扩增原代结膜干细胞 (CjSCs)
发布: 2022年12月20日第12卷第24期 DOI: 10.21769/BioProtoc.4569 浏览次数: 2172
评审: Vivien Jane Coulson-ThomasSudhir VermaAnonymous reviewer(s)
Abstract
Conjunctival disorders are multivariate degenerative ocular surface diseases that can jeopardize ocular function and impair visual capacity in severe cases. The recent development of stem cell technologies has shed a new light on the treatment of conjunctival disorders as the regenerative medicine using endogenous stem cells becomes a potential therapeutic strategy. However, the efficient in vitro expansion of the endogenous stem cells dominating the conjunctival regeneration, the conjunctival stem cells (CjSCs), remains challenging. Existing protocols largely adopted primary culture using feeder layers, which has limited efficiency and risk of contamination. Here, we report a protocol for the isolation and expansion of primary CjSCs derived from human or animal tissues. This protocol adopts collagenase-based enzymatic digestion to release the primary cells from conjunctival tissues and utilizes a feeder-free culture strategy based on a small molecule inhibitor cocktail that stimulates the expansion of CjSCs. The CjSCs generated with this method were rapidly dividing and highly homogeneous. They also expressed characteristic stem cell markers and exhibited differentiation potency. These findings marked an important step forward in building stable CjSCs in vitro expansion, which will help researchers better understand the biology of ocular surface stem cells and develop innovative regenerative medicine approaches for ocular surface diseases.
Graphical abstract
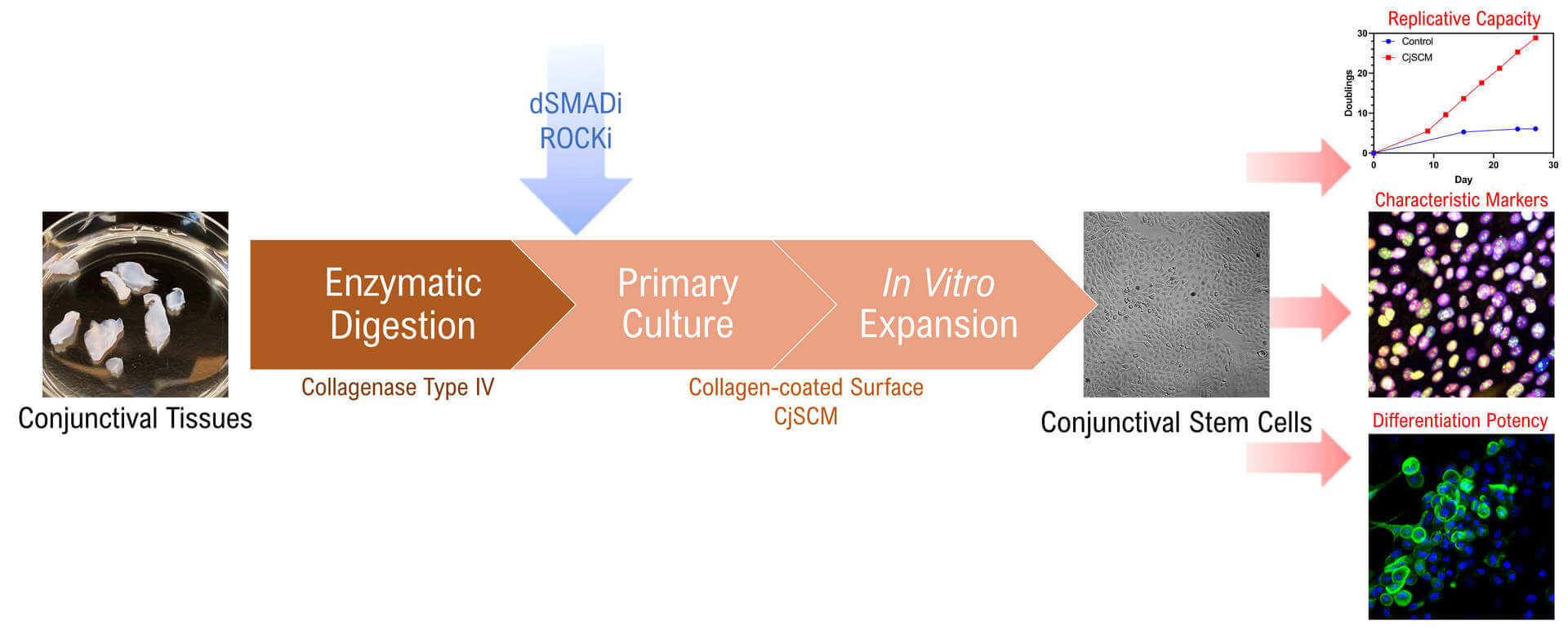
Background
The conjunctiva is a significant part of the ocular surface that functions as the immune barrier and protects the integrity of the eyes (Nguyen et al., 2011; Gipson, 2016). Similar to the involuted epithelium on gastrointestinal and airway internal surfaces, it is comprised of nonkeratinized mucosal epithelium containing mucin-secreting goblet cells, which provide the fundamental support of the tear film as well as the homeostasis of the ocular surface (Barabino et al., 2012). Disorders including cicatrizing conjunctivitis, dry eye diseases, and Stevens-Johnson syndrome can disrupt the normal function of the conjunctiva, which could further damage the ocular surface and jeopardize the vision (Barabino et al., 2003; Kohanim et al., 2016). Although these conditions are threatening millions of patients worldwide, existing treatments based on pharmaceutical therapy and amniotic membrane transplantation are limited by mediocre efficacy and insufficient regeneration (Liu et al., 2010; Tseng et al., 2016).
Recent advances in stem cell technology and regenerative medicine have made stem cell transplantation an alternative strategy for treating ocular surface diseases, and a huge interest has been raised in studying the endogenous stem cells residing in the conjunctiva, the conjunctival stem cells (CjSC) (Ramos et al., 2015; Nakamura et al., 2016; Williams et al., 2018; Zhong et al., 2021a). These are the bipotent progenitor that give rise to both conjunctival keratinocytes and conjunctival goblet cells, thus holding tremendous potential in conjunctival regeneration (Pellegrini et al., 1999; Majo et al., 2008; Nomi et al., 2021). However, as a critical premise in developing CjSC-based applications, the primary culture and in vitro expansion of CjSCs remains challenging. Early studies largely utilized feeder layers to support the primary culture of CjSCs, while the later ones tended to adopt the feeder-free culture system supplemented with cytokines and small molecules targeting key signaling pathways (Pellegrini et al., 1999; Stewart et al., 2015; Wu et al., 2020). The latest studies have demonstrated the efficacy of small molecule–based dual SMAD signaling inhibition (dSMADi) and ROCK signaling inhibition (ROCKi) in culturing epithelial stem cells derived from pulmonary alveoli, esophagus, and intestine (Mou et al., 2016; Zhang et al., 2018). Dual SMAD signaling (TGFβ and BMP signaling pathways) has been proved to regulate the maturation, self-renewal, and quiescence of epithelial stem cells, while ROCK signaling pathway contributes to the mechanotransduction and cell cycle progression (Kobielak et al., 2007; Amano et al., 2010; Tata et al., 2013). Therefore, we hypothesize that the combination of dSMADi and ROCKi can be applied to the CjSC culture and stimulate expansion.
Here, we established an isolation and feeder-free culture method for CjSCs derived from both human and animal conjunctival tissues. The primary conjunctival epithelial cells were first isolated through enzymatic digestion and seeded on a collagen-coated surface. Then, the cells were subjected to primary culture with the conjunctival stem cell medium (CjSCM) encompassing a small molecule cocktail of dSMADi and ROCKi, which stimulated the outgrowth of the stem cell population. CjSCM outperformed the control medium in generating the cells with a higher replicative capacity and shorter doubling time. The cells cultured with CjSCM also showed upregulated expression of stemness and lineage markers while retaining the differentiation potency. This method can be applied to produce functional CjSCs and support the development of regenerative medicine and stem cell therapy for ocular surface diseases.
Materials and Reagents
Sterilization pouches
BD syringe needle (BD, catalog number: 230-45094)
100 mm TC-treated culture dish (Corning, catalog number: 430167)
Costar® 6-well plates (Corning, catalog number: 3516)
Costar® 12-well plates (Corning, catalog number: 3513)
FalconTM 70 μm cell strainers (Corning, catalog number: 08-771-2)
Microcentrifuge tubes (ThermoFisher Scientific, catalog number: 3448PK)
15 mL conical centrifuge tubes (ThermoFisher Scientific, catalog number: 339651)
50 mL conical centrifuge tubes (ThermoFisher Scientific, catalog number: 339653)
Millicell EZ SLIDE 8-well glass, sterile (Sigma-Aldrich, catalog number: PEZGS0816)
Phosphate buffer solution (PBS) (ThermoFisher Scientific, catalog number: 10010023)
Dulbecco's modified Eagle medium (DMEM) (ThermoFisher Scientific, catalog number: 11885084)
DMEM/F12 medium (ThermoFisher Scientific, catalog number: 11330032)
Penicillin-streptomycin (pen-strep) (ThermoFisher Scientific, catalog number: 15140122)
0.25% trypsin-EDTA (ThermoFisher Scientific, catalog number: 25200056)
Fetal bovine serum (FBS) (ThermoFisher Scientific, catalog number: 10082147)
Keratinocyte serum-free medium (KSFM) with bovine pituitary extract (BPE) (ThermoFisher Scientific, catalog number: 17005042)
Collagen I, bovine (ThermoFisher Scientific, catalog number: A1064401)
Insulin-transferrin-selenium (ITS-G) (ThermoFisher Scientific, catalog number: 41400045)
4% paraformaldehyde solution (ThermoFisher Scientific, catalog number: J19943.K2)
Triton X-100 (ThermoFisher Scientific, catalog number: A16046.0F)
Fluoromount-GTM mounting medium (ThermoFisher Scientific, catalog number: 00-4958-02)
TRIzolTM reagent (ThermoFisher Scientific, catalog number: 15596026)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9418)
DAPI (Sigma-Aldrich, catalog number: D9542)
Hydrocortisone (Sigma-Aldrich, catalog number: H0888)
Cholera toxin (Sigma-Aldrich, catalog number: C8052)
3,3′,5-Triiodo-L-thyronine sodium salt (Sigma-Aldrich, catalog number: T6397)
Recombinant human EGF protein (R&D Systems, catalog number: 236-EG)
Recombinant human BMP-4 (BioLegend, catalog number: 795606)
Recombinant human KGF (BioLegend, catalog number: 711702)
Recombinant human IL-13 (BioLegend, catalog number: 571106)
Recombinant human BMP-4 (BioLegend, catalog number: 795606)
Y-27632 dihydrochloride (Tocris, catalog number: 1254)
A83-01 (Tocris, catalog number: 2939)
DMH-1 (Tocris, catalog number: 4126)
Collagenase IV, powder (ThermoFisher Scientific, catalog number: 17104019)
Direct-zolTM RNA purification miniprep kit (Zymo Research, catalog number: R2050)
ProtoScript® II First Strand cDNA synthesis kit (New England Biolabs, catalog number: E6560L)
Luna® Universal qPCR master mix (New England Biolabs, catalog number: M3003L)
Conjunctival stem cell medium (CjSCM) (see Recipes)
Control medium (see Recipes)
Goblet cell differentiation medium (see Recipes)
DMEM/F12 + pen-strep (see Recipes)
DMEM/F12 + pen-strep + FBS (see Recipes)
0.5% type IV collagenase solution (see Recipes)
Equipment
Tweezers (Dumont, catalog number: 0203-54-PO)
Surgical scissors (FST, catalog number: 15011-12)
Hemocytometer (ThermoFisher Scientific, catalog number: 02-671-51B)
Pipette sets (Eppendorf, catalog number: 2231300004)
Centrifuge (Eppendorf, model: 5810R)
Pipet-aid (Drummond Scientific, catalog number: 4-000-101)
Biosafety cabinet (Labconco, model: 3460009)
Orbital shaker (Benchmark, model: BT4001)
Water bath (Fisher Scientific, model: 210)
Cell culture incubator (VWR, model: 51014992)
NanoDropTM 2000 spectrophotometer (ThermoFisher Scientific, catalog number: ND-2000)
Microcentrifuge (ThermoFisher Scientific, catalog number: 75002492)
StepOne M real-time PCR system (ThermoFisher Scientific, catalog number: 4376357)
Confocal microscope (Leica, model: SP8)
Autoclave machine (Tuttnauer, model: EZ9)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Zhong, Z. and Chen, S. (2022). Isolation and Expansion of Primary Conjunctival Stem Cells (CjSCs) from Human and Rabbit Tissues. Bio-protocol 12(24): e4569. DOI: 10.21769/BioProtoc.4569.
分类
细胞生物学 > 细胞分离和培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









