- EN - English
- CN - 中文
Babesia duncani in Culture and in Mouse (ICIM) Model for the Advancement of Babesia Biology, Pathogenesis and Therapy
促进巴贝虫生物学、发病机制和治疗的巴贝虫培养和小鼠(ICIM)模型
发布: 2022年11月20日第12卷第22期 DOI: 10.21769/BioProtoc.4549 浏览次数: 1926
评审: Kristin L. ShinglerWenn-Chyau LeeLucy XieAnonymous reviewer(s)
Abstract
Babesiosis is a tick-borne disease caused by pathogens belonging to the genus Babesia. In humans, the disease presents as a malaria-like illness and can be fatal in immunocompromised and elderly people. In the past few years, human babesiosis has been a rising concern worldwide. The disease is transmitted through tick bite, blood transfusion, and transplacentally in rare cases, with several species of Babesia causing human infection. Babesia microti, Babesia duncani, and Babesia divergens are of particular interest because of their important health impact and amenability to research inquiries. B. microti, the most commonly reported Babesia pathogen infecting humans, can be propagated in immunocompetent and immunocompromised mice but so far has not been successfully continuously propagated in vitro in human red blood cells (hRBCs). Conversely, B. divergens can be propagated in vitro in hRBCs but lacks a mouse model to study its virulence. Recent studies have highlighted the uniqueness of B. duncani as an ideal model organism to study intraerythrocytic parasitism in vitro and in vivo. An optimized B. duncani in culture and in mouse (ICIM) model has recently been described, combining long-term continuous in vitro culture of the parasite in human red blood cells with an animal model of parasitemia (P) and lethal infection in C3H/HeJ mice. Here, we provide a detailed protocol for the use of the B. duncani ICIM model in research. This model provides a unique and sound foundation to gain further insights into the biology, pathogenesis, and virulence of Babesia and other intraerythrocytic parasites, and has been validated as an efficient system to evaluate novel strategies for the treatment of human babesiosis and possibly other parasitic diseases.
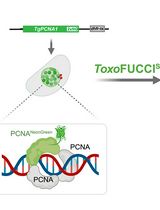
Graphical abstract:
ICIM model [Adapted and modified from Pal et al. (2022)]
Background
Babesiosis is an emerging tick-borne disease caused by apicomplexan parasites of the genus Babesia. Like the other apicomplexan parasite Plasmodium falciparum, the causative agent of human malaria, Babesia parasites also invade human red blood cells (hRBCs) to cause the pathological symptoms associated with human babesiosis, with clinical outcomes ranging from mild to severe and, in some cases, leading to death. More than 100 species of Babesia are known to cause infection in a wide range of mammalian hosts, including livestock, with significant health and economic impacts (Renard and Ben Mamoun, 2021). Human babesiosis is an emerging worldwide concern; although humans are not the natural host of Babesia. Over the past 50 years there has been a rapid increase in cases of human babesiosis caused by different Babesia species (Renard and Ben Mamoun, 2021). While B. microti is the most common species causing human babesiosis, other species such as B. divergens and B. duncani have also been shown to lead to severe and sometimes lethal clinical outcomes (Persing et al., 1995; Rożej-Bielicka et al., 2015; Vannier et al., 2015; Kumar et al., 2021; Renard and Ben Mamoun, 2021). The first case of human babesiosis was identified in a splenectomized patient in Europe, but most cases of babesiosis found in northeastern and midwestern United States had no history of immune impairment (Skrabalo and Deanovic, 1957; Hunfeld et al., 2008; Vannier et al., 2015). Although humans are a dead-end host of Babesia parasites, cases of accidental transmission through blood transfusion from Babesia-infected individuals have been reported, and some rare cases of transplacental transmission have also been documented (Fox et al., 2006, Walker et al., 2022). The first case of transfusion-transmitted babesiosis (TTB) was reported in 1979, ten years after the first reported clinical case of B. microti human babesiosis in the United States (Scholtens et al., 1968, Jacoby et al., 1980). Although B. microti is the most common agent of TTB, cases caused by B. duncani have also been reported (Kjemtrup and Conrad, 2000; Kjemtrup et al., 2002). As the recipients are often immunocompromised, TTB could be fatal (Herwaldt et al., 1997; Claycomb et al., 1998; Conrad, 2000; Leiby, 2011; Renard and Ben Mamoun, 2021). The blood donors carrying Babesia parasites are often asymptomatic, which highlights the necessity for generating tools for efficient diagnosis of parasite infection and for the development of a vaccine and new therapies for the prevention and treatment of human babesiosis. For a long time, the life cycle and biology of Babesia parasites have been poorly elucidated, mainly due to the lack of suitable tools for continuous in vitro propagation of the parasites and/or lack of animal models to study their pathogenesis and virulence. The in culture and in mouse (ICIM) model for Babesia infection described herein will assist in answering some key questions about Babesia biology, pathogenesis, and survival in human red blood cells, namely how these parasites interact with the host and modulate its immune response. The ICIM model has also been important in the discovery and development of novel antibabesial drugs (Lawres et al., 2016; Chiu et al., 2021; Pal et al., 2022). In previous studies (Abraham et al., 2018), the continuous in vitro culture of B. duncani in hRBCs was reported in commercially available Claycomb (Sigma) and HL1 (Lonza) media. The HL1 medium has been discontinued since March 2021; the Claycomb medium often suffers supply shortages, is relatively expensive, and contains several mammalian proteins (bovine albumin, fetuin, transferrin, human insulin, long R3IGF-1, and long EGF) that, while important for HL-1 cardiomyocytes, are of no importance to Babesia intraerythrocytic development (Claycomb et al., 1998; White et al., 2004; Singh et al., 2022). We have recently reported that the DMEM/F-12 is an alternative growth medium for continuous in vitro culture of B. duncani in hRBCs (Singh et al., 2022). It differs from a standard DMEM medium, which does not support the growth of the parasite, by the presence of six amino acids (alanine, asparagine, aspartic acid, cysteine, glutamic acid, and proline), two vitamins (biotin and cobalamin), and four inorganic salts (cupric sulfate, ferric sulfate, magnesium chloride, and zinc sulfate) as well as hypoxanthine, thymidine, linoleic acid, lipoic acid, and putrescine (Singh et al., 2022). Furthermore, an optimized animal model of B. duncani infection that allows a consistent and reproducible evaluation of parasite development and virulence in mice has been recently described (Pal et al., 2022). The ICIM model, which combines the in vitro propagation of the parasite in human red blood cells and parasite virulence in mice, will usher a new era of advanced research on Babesia by facilitating the use of genetic tools and resources to conduct large scale functional analysis to link gene expression and function to disease progression and parasite virulence. This model will greatly enhance our understanding of this disease, as well as help in developing novel therapeutic strategies with improved efficacy.
Materials and Reagents
For in vitro culture
6-well plate (Corning, catalog number: 353046)
1.7 mL microcentrifuge tubes (Thomas Scientific, catalog number: 1149K01)
Centrifuge tubes (Corning, Falcon tubes, catalog numbers: 430829 [50 mL], 352096 [15 mL])
Pipette tips [USA Scientific, catalog numbers: 1121-3810 (10 μL), 1120-8810 (200 μL), 1111-2721 (1,000 μL)]
Plugged serological sterile pipettes [Corning, Falcon pipettes, catalog numbers: 357543 (5 mL), 357551 (10 mL), 357525 (25 mL)]
Millex filter units [Millipore, catalog numbers: SLGSR33SS (Syringe driven), S2GPU10RE (vacuum driven)]
Pipettes [Eppendorf, catalog numbers: K24694J (1,000 μL), J46084J (200 μL), H16607J (20 μL), I54818J (10 μL)]
Pipette AID (Drummond Scientific)
Aspiration pipettes (Santa Cruz, catalog number: 357781)
DMEM/F-12 (Lonza, catalog number: BE04-687/U1, Basel, Switzerland; or Thermo Fisher Scientific, catalog number: 21331020)
DMEM (Thermo Fisher Scientific, Gibco, catalog number: 11-966-025)
RPMI (Thermo Fisher Scientific, Gibco, catalog number: 11-875-093)
FBS (Thermo Fisher Scientific, Gibco, catalog number: 10438-026, Waltham, MA, USA)
50× HT media supplement Hybrid-MaxTM (Sigma, catalog number: H0137)
L-glutamine (Thermo Fisher Scientific, Gibco, catalog number: 25030-081)
Antimycotic (antibiotic) (Thermo Fisher Scientific, Gibco, catalog number: 15240-062)
Gentamicin reagent solution (Thermo Fisher Scientific, Gibco, catalog number: 15710-072)
A+ human RBCs (American Red Cross or Interstate Blood Bank, Inc.)
B. duncani WA1 strain (BEI Resources, NR-12311)
DMEM/F-12 complete media (250 mL) (see Recipes)
For cryopreservation
Glycerolyte 57 solution (Baxter Healthcare corporation, Deerfield, IL, USA, catalog number: 4A7831)
Cryotube vials (Thermo Scientific, catalog number: 363401)
For slide preparation
Premium microscope slides (Fisherfinest, catalog number: 22038-103)
Hemacolor solution I, fixative (Sigma-Aldrich, catalog number: 65044A-85)
Hemacolor solution II (Sigma-Aldrich, catalog number: 65044B-85)
Hemacolor solution III (Sigma-Aldrich, catalog number: 65044C)
Immersion oil (Cargille, catalog number: 16482)
For mouse infection
BD PrecisionGlideTM 27G needle (BD, catalog number: 305109)
Lithium heparin–coated blood collection tube (McKesson 574507, Greiner Bio-One, MiniCollect, catalog number: 450477)
Heparinized capillary tubes (Fisher Scientific, catalog number: 22-260-950)
Isoflurane (Covetrus, catalog number: 11695-6777-2)
PEG 400 (Thermo Fisher Scientific, Avantor J.T. Baker U216-07, catalog number: 02-003-646)
1× PBS diluted from 10× PBS pH 7.4 (Gibco, catalog number: 70011-044)
Mouse strains: C3H/HeJ (from Jackson Laboratories, Bar Harbor, ME)
Note: Our studies have shown that C3H/HeJ mice are susceptible to B. duncani infection following IV inoculation with doses of infected RBCs (iRBCs) ranging between 102 and 107. We found that 107 and 106 iRBC doses elicit an acute increase in parasitemia (P) (up to 35%) within a short span of time [3–5 days post infection (DPI)], whereas mice inoculated with doses of 102–105 iRBCs show a delayed onset of infection with a lower peak P (Pal et al., 2022). Parasitemia levels in C3H/HeJ mice were always higher in females compared to males at the same infection dose (Aguilar-Delfin et al., 2001, Pal et al., 2022). In contrast, C57BL/6J mice showed 100% survival with no detectable P following inoculation with 104 B. duncani–iRBC. Infection of Balb/cJ with 104 B. duncani–iRBC resulted in increased P over time in all mice; however, approximately 50% of the mice cleared the infection and survived, whereas the other half continued to show detectable P and succumbed to infection (Pal et al., 2022).
30% isoflurane (50 mL) (inhalation anesthetic; see Recipes)
Equipment
Sorvall legend XTR centrifuge (Thermo Scientific, catalog number: 75004521)
Microscope (Nikon Eclipse 5Oi)
SterilGARD III advance biological safety cabinet (The Baker Company, catalog number: SG603)
Water bath (Fisher brand, model: FSGPD15D)
Tri gas incubator (Thermo Scientific, model: HERACELL VIOS 160i)
Eppendorf MiniSpin (Millipore-Sigma, catalog number: EP022620100)
Software
GraphPad Prism version 9.4.1
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Kumari, V., Pal, A. C., Singh, P. and Ben Mamoun, C. (2022). Babesia duncani in Culture and in Mouse (ICIM) Model for the Advancement of Babesia Biology, Pathogenesis and Therapy. Bio-protocol 12(22): e4549. DOI: 10.21769/BioProtoc.4549.
分类
微生物学 > 微生物细胞生物学 > 细胞分离和培养
微生物学 > 体内实验模型 > 原生动物
细胞生物学 > 细胞分离和培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link