- EN - English
- CN - 中文
Measuring Intracellular H2O2 in Intact Human Cells Using the Genetically Encoded Fluorescent Sensor HyPer7
使用基因编码荧光传感器 HyPer7 测量完整人体细胞中的双氧水水平
发布: 2022年10月20日第12卷第20期 DOI: 10.21769/BioProtoc.4538 浏览次数: 3347
评审: David PaulVsevolod BelousovAnonymous reviewer(s)
Abstract
Depending on its local concentration, hydrogen peroxide (H2O2) can serve as a cellular signaling molecule but can also cause damage to biomolecules. The levels of H2O2 are influenced by the activity of its generator sites, local antioxidative systems, and the metabolic state of the cell. To study and understand the role of H2O2 in cellular signaling, it is crucial to assess its dynamics with high spatiotemporal resolution. Measuring these subcellular H2O2 dynamics has been challenging. However, with the introduction of the super sensitive pH-independent genetically encoded fluorescent H2O2 sensor HyPer7, many limitations of previous measurement approaches could be overcome. Here, we describe a method to measure local H2O2 dynamics in intact human cells, utilizing the HyPer7 sensor in combination with a microscopic multi-mode microplate reader.
Graphical abstract:
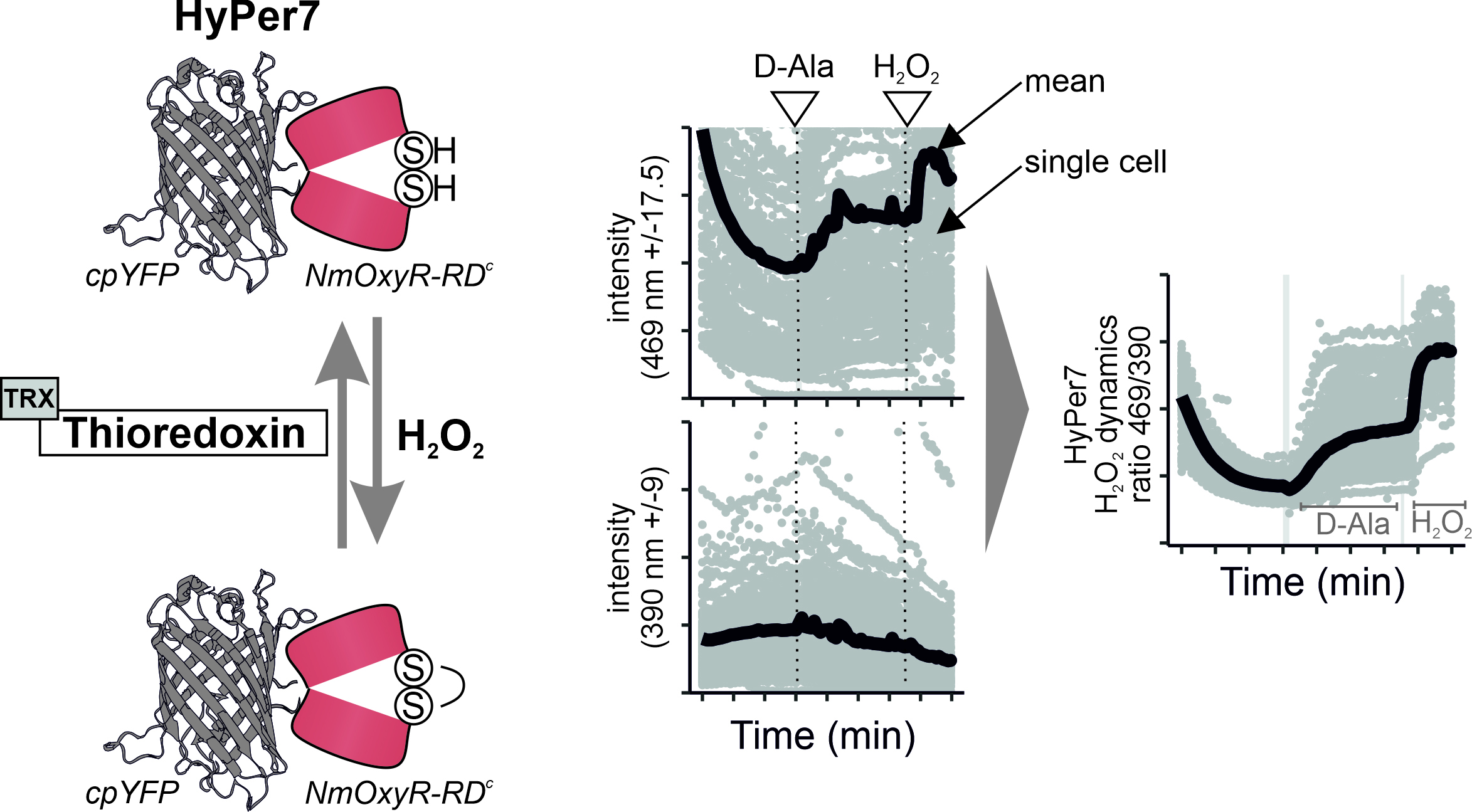
Overview of HyPer7 sensor function and measurement results.
Background
Aerobic life comes at a price due to the formation of reactive oxygen species (ROS) as an unavoidable by-product of essential metabolic processes. ROS is a collective term for reactive chemical species containing oxygen, of which hydrogen peroxide (H2O2) is the most relevant member due to its long half-life and comparably high cellular concentration. H2O2 has both beneficial and deleterious effects on cells depending on its levels, flux, and the functional state of the cell (Sies and Jones, 2020). Consequently, cellular H2O2 levels are tightly controlled.
The subcellular dynamics of H2O2 are poorly understood in intact mammalian cells. To explore these dynamics, biosensors that are targetable, sensitive, specific, and reversible are required. Research previously relied on small chemical probes (mostly irreversible and therefore not measurements of dynamic fluxes of H2O2) or comparably insensitive genetically encoded sensors (reversible but insensitive) (Roma et al., 2018; Kalinovic et al., 2019; Liao et al., 2020; Plecita-Hlavata et al., 2020).
In recent years, more sensitive genetically encoded fluorescent H2O2 sensors were developed, including HyPer7 (Pak et al., 2020). HyPer7 consists of a circular permutated yellow fluorescent protein (cpYFP) that is integrated into the H2O2-sensing regulatory domain of Neisseria meningitidis OxyR (Oxy-RD). OxyR is a natural H2O2 sensor and transcription factor. OxyR from different organisms have different sensitivities; the specific Oxy-RD domain from N. meningitidis renders HyPer7 particularly sensitive and allows measurement of close to baseline H2O2 levels in otherwise unperturbed mammalian tissue cell lines.
Oxy-RD contains two cysteine residues that react in a sensitive and highly specific manner with H2O2 and consequently form a disulfide bond. This disulfide bond in Oxy-RD puts a strain on the fused cpYFP, thus changing the cpYFP excitation spectrum. cpYFP has two excitation maxima at ca 400 nm and 499 nm, respectively, and emits at 516 nm. Changes in the sensor oxidation state result in ratiometric changes with decreases in the excitation maximum at 400 nm and increases at 499 nm upon Oxy-RD oxidation (Pak et al., 2020). Notably, targeted mutations in the cpYFP backbone result in pH insensitivity of HyPer7. This is a clear advantage compared with previous HyPer sensor variants (e.g., HyPer3) that were all pH sensitive in the physiological pH range and required laborious pH control experiments.
Sensors for different biomolecules can be combined with different treatments of cells, with different genetic backgrounds, or with genetic engineering tools. One such genetically encoded tool is D-amino acid oxidase (DAO), an enzyme that converts D-amino acids to the corresponding α-keto acid and generates as a by-product H2O2 (Matlashov et al., 2014). Since DAO can be targeted to different regions within a cell, it can be employed for the localized generation of H2O2.
Here, we describe a protocol to measure local H2O2 dynamics in cytosol and mitochondria. Combining this method with genetically engineered tools, such as the DAO system or CRISPR-cas9–mediated gene editing, to specifically remove parts of the antioxidative system allows detailed investigations of subcellular H2O2 dynamics.
Materials and Reagents
Cultivation of HEK293 cells
1.5 mL Eppendorf tube (Diagonal, catalog number: 02-023-0100)
Sterile filter pipette tips:
10 µL (Greiner Bio-One, Sapphire, catalog number: 772353)
100 µL (Greiner Bio-One, Sapphire, catalog number: 774353)
1,250 µL (Greiner Bio-One, Sapphire, catalog number: 778353)
96-well plate (µClear, Greiner Bio-One, catalog number: 655090)
15 mL Falcon tube (VWR, catalog number: 734-0451)
LUNA reusable cell counting slide (Logos Biosystems, catalog number: L12011)
Autoclaved disposable glass Pasteur pipettes without cotton pad (VWR, catalog number: HECH40567001)
Sterile, individually packed serological pipettes:
5 mL pipettes (Sarstedt, catalog number: 86.1253.001)
10 mL pipettes (Sarstedt, catalog number: 86.1254.001)
10% Fetal calf serum (FCS, Sigma-Aldrich, catalog number: F0804)
1% Penicillin/streptomycin (P/S, Sigma-Aldrich, catalog number: P0781-100ML)
Dulbecco's Modified Eagle Medium high glucose (DMEM, Thermo Fisher, Gibco, catalog number: 41965062)
Reagent reservoir (VWR, catalog number: 613-1175)
Flp-In T-Rex HEK293 cells (Human embryonic kidney 293, Invitrogen, catalog number: R78007)
Poly-L-lysine (Sigma-Aldrich, catalog number: P4832-50mL)
Dulbecco's Phosphate Buffered Saline powder (DPBS, Sigma-Aldrich, catalog number: D5652)
10× Trypsin-EDTA solution (Sigma-Aldrich, catalog number: T4174)
Trypan Blue (Logos Biosystems, catalog number: T13001)
DMEM medium-complete (Gibco, catalog number: 41965039) (see Recipes)
DPBS (see Recipes)
Trypsin-EDTA solution (see Recipes)
Transfection of HEK293 cells
Plasmids:
pCS2+HyPer7-NES [addgene: plasmid #136467 (Pak et al., 2020)]
pCS2+MLS-HyPer7 [addgene: plasmid #136470 (Pak et al., 2020)]
Polyethylenimine (PEI) (Polysciences, catalog number: 23966-1)
Polyethylenimine (PEI) 1 mg/mL (see Recipes)
Dulbecco’s Modified Eagle Medium medium-pure (DMEM, Thermo Fisher, Gibco, catalog number: 41965062) (see Recipes)
Induced expression of DAO in HEK293 cells
Doxycycline (DOX) (AppliChem, catalog number: A2951,0005)
H2O2 measurement
D-Alanine (Sigma-Aldrich, catalog number: 338-69-2)
L-Alanin BioChemica (Applichem, catalog number: A3690,0100)
H2O2 (Sigma-Aldrich, catalog number: 216763-100ML)
Minimal media (see Recipes):
NaCl (Roth, catalog number: 7647-14-5)
KCl (Roth, catalog number: 7447-40-7)
MgCl2 (Roth, catalog number: 2189.2)
CaCl2 (Merck, catalog number: 23.891.000)
HEPES (VWR, catalog number: 7365-45-9)
Glucose (CIL, catalog number: 110187-42-3)
10% Fetal calf serum (FCS, Sigma-Aldrich, catalog number: F0804)
Equipment
Cultivation of HEK293 cells
Laminar flow hood class II (ENVAIR eco)
CO2 incubator (New Brunswick)
Vacuum pump (laboport)
Microscope (motic AE2000)
Multichannel pipette (VWR)
Cell counter – LUNA-II (Logos Biosystems, catalog number: L40002)
Equipped cell culture laboratory containing, e.g., a laminar flow hood class II (ENVAIR eco), a CO2 incubator for cultivation of cells (with cultivation set at 37 °C and 5% CO2), a vacuum pump for removal of medium using sterile glass Pasteur pipettes, a microscope, a cell counter, and a cooling centrifuge.
Transfection of HEK293 cells
In addition to A.
Vortex shaker
H2O2 measurement
Cytation 3 (Agilent, BioTek)
CO2 control
Injection system (optional)
390LED Rev H (Agilent, BioTek, catalog number: 1225009)
roGFPsmall filterblock ex.390 em. 525 Rev D (Agilent, BioTek, catalog number: 1225108)
465LED Rev I (Agilent, BioTek, catalog number: 1225001)
GFP filterblock ex. 469 em. 525 Rev I (Agilent, BioTek, catalog number: 1225101)
Minimal media [HEPES buffer (HBSS) solution from (Poburko et al., 2011)] (see Recipes)
Complete minimal media [HEPES buffer (HBSS) solution from (Poburko et al., 2011)] (see Recipes)
Software
Redox Ratio Analysis (RRA) [Dr M.D. Fricker, https://markfricker.org/77-2/software/redox-ratio-analysis/ (Fricker, 2016)]
Rstudio (https://www.rstudio.com/)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Jacobs, L. J. H. C., Hoehne, M. N. and Riemer, J. (2022). Measuring Intracellular H2O2 in Intact Human Cells Using the Genetically Encoded Fluorescent Sensor HyPer7. Bio-protocol 12(20): e4538. DOI: 10.21769/BioProtoc.4538.
分类
细胞生物学 > 细胞成像 > 活细胞成像
生物化学 > 其它化合物 > 活性氧
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link