- EN - English
- CN - 中文
Measurement of Ascorbate Peroxidase Activity in Sorghum
高粱中抗坏血酸过氧化物酶活性的测定
发布: 2022年10月20日第12卷第20期 DOI: 10.21769/BioProtoc.4531 浏览次数: 3881
评审: Khyati Hitesh ShahPrasad S. DalviAnonymous reviewer(s)
Abstract
The ascorbate peroxidase (APX) is a widely distributed antioxidant enzyme. It differs from catalase and other peroxidases in that it scavenges/reduces reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) to water using reduced ascorbate as the electron donor. It is advantageous over other similar antioxidant enzymes in scavenging ROS since ascorbate may react with superoxide, singlet oxygen, and hydroxyl radical, in addition to reacting with H2O2. The estimation of its activity is helpful to analyze the level of oxidative stress in living systems under stressful conditions. The present protocol was performed to analyze the impact of heavy metal chromium (Cr) toxicity on sorghum plants in the form of APX enzyme activity under the application of glycine betaine (GB) and arbuscular mycorrhizal fungi (AMF) as stress ameliorators. Plant defense strategies against heavy metals toxicity involve the utilization of APX and the instigation of AMF symbiotic system, as well as their possible collaboration with one another or with the plant antioxidant system; this has been examined and discussed in literature. In this protocol, an increased APX activity was observed on underlying functions and detoxification capabilities of GB and AMF that are typically used by plants to enhance tolerance to Cr toxicity.
Graphical abstract:
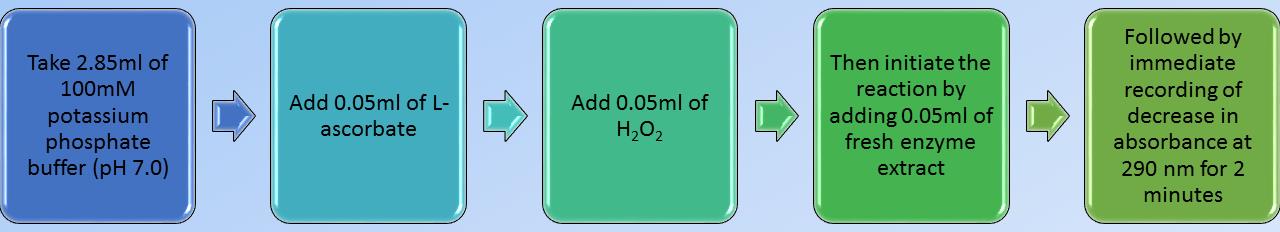
Flow chart of standardized or calibrated enzyme assay with leaf samples of sorghum
Background
Ascorbate peroxidase (APX) is an antioxidant enzyme, involved in the removal of toxic components such as reactive oxygen species (ROS) produced during physiologic and metabolic activities of the cell. Cells need to detoxify ROS efficiently as a consequence of abiotic stresses. A complex enzymatic antioxidative system could be developed by cells, which controls the production of ROS and ultimately protects the plant against oxidative damage (Ashraf et al., 2015). This metal-induced plant anti-oxidative defense mechanism is differential and largely based on the types of heavy metals and plant species. Elevated anti-oxidative enzyme activities attenuate abiotic oxidative stress and play a pivotal role in plants adapting against various environmental stresses (Singh et al., 2008). Furthermore, a decline in APX activity suggests that higher Cr toxicity might be inhibiting its ROS-eliminating role in plants. Decreased APX activity with increased metal toxicity was previously reported in Indian mustard (Mobin and Khan, 2007), oilseed rape (B. Ali, et al., 2014), and wheat (S. Ali, et al., 2015). Overall, increasing Cr toxicity triggers antioxidant production (mainly responsible for ROS quenching), representing plants’ potential to withstand Cr-polluted soils up to a certain level, which is further dependent on plant species and environmental conditions. Anjum et al. (2017) reported that at lower heavy metals concentrations the activity of antioxidant enzymes increased, whereas at higher concentrations the activity did not further increase. Samantaray et al. (1999) used peroxidase and catalase activities as enzyme markers for Cr-tolerant mung bean cultivars. The observed increase in antioxidant enzyme activity might have been in direct response to the generation of superoxide radicals by Cr-induced blockage of the electron transport chain in the mitochondria. Higher increases observed due to Cr(VI) indicated that its addition probably generated more singlet oxygen than Cr(III). The decrease in enzyme activity, as the concentration of external Cr increases, might be due to the inhibitory effect of Cr ions on the enzyme system itself.
In the present study, a comprehensive account of past developments and current trends using glycine betaine (GB) and arbuscular mycorrhizal fungi (AMF) in the research on Cr toxicity and its amelioration in sorghum plants has been attempted. Moreover, another line of plant defense strategy against heavy metals toxicity, which involves the utilization of APX and the instigation of AMF symbiotic system, as well as their possible collaboration with one another or with the plant antioxidant system, has been examined and discussed (Kumar, 2021). The study focuses on the underlying functions and detoxification capabilities of GB and AMF that are typically used by plants to enhance tolerance to Cr toxicity.
Materials and Reagents
Aluminum foil
Hydrogen peroxide, 35% in water (H2O2) (TCI, catalog number: H1222, CAS RN: 7722-84-1)
L(+)-ascorbic acid (CAS 50-81-7) (Merck Millipore, catalog number:100468)
0.1 M potassium phosphate buffer (pH 7.0) (see Recipes)
Reaction mixture (see Recipes)
Equipment
Scale
Pipettes
Stirrer
Mortar and pestle
Centrifuge machine
Double beam UV-VIS spectrophotometer with Graphic LCD – Typr 2205
Glassware: test tubes, beakers, conical flask, measuring cylinder, and a volumetric flask (Slisco scientific and Ace Glass Incorporated)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Kumar, P. (2022). Measurement of Ascorbate Peroxidase Activity in Sorghum. Bio-protocol 12(20): e4531. DOI: 10.21769/BioProtoc.4531.
分类
植物科学 > 植物生物化学 > 代谢物
植物科学 > 植物生理学 > 非生物胁迫
生物化学 > 其它化合物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link