- EN - English
- CN - 中文
Assay for Protealysin-like Protease Inhibitor Activity
蛋白酶样蛋白酶抑制剂活性测定
发布: 2022年10月05日第12卷第19期 DOI: 10.21769/BioProtoc.4528 浏览次数: 2160
评审: Anonymous reviewer(s)
Abstract
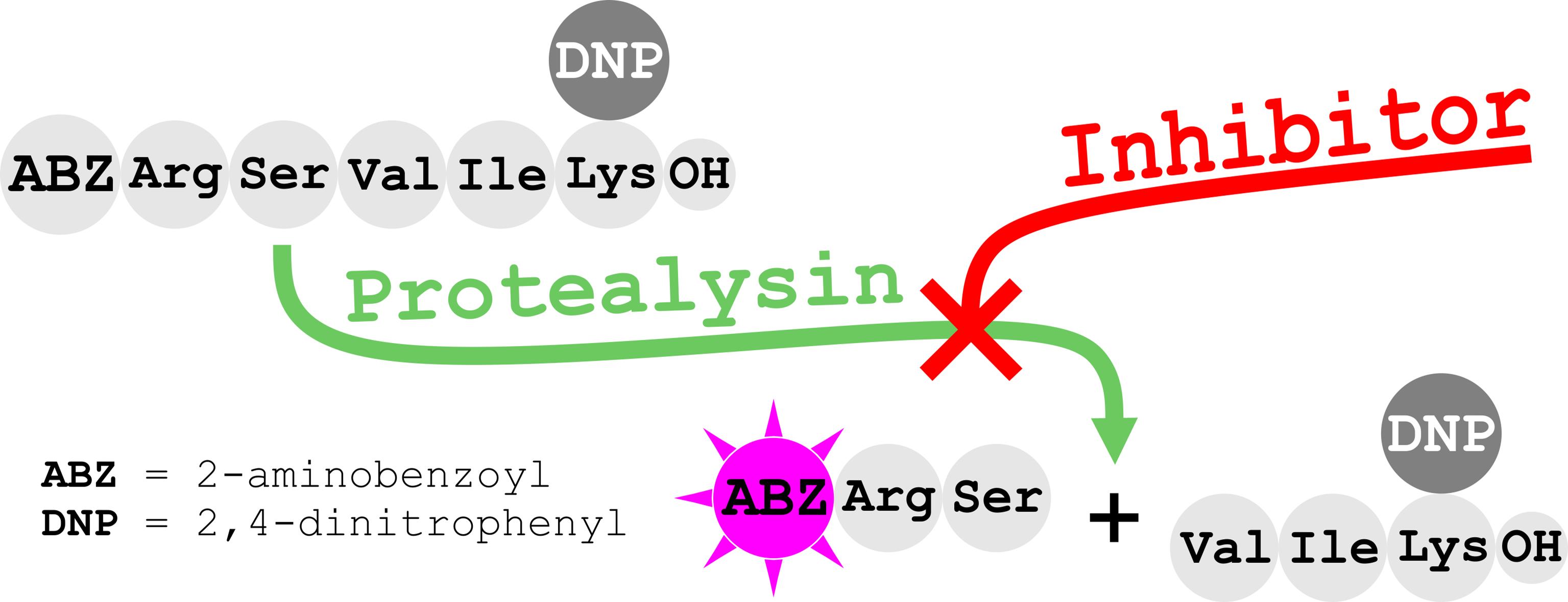
Here, we present the first quantitative method for the activity analysis of protealysin-like protease (PLP) inhibitors. This approach is based on a previously developed method for protealysin activity determination by hydrolysis of internally quenched fluorescent peptide substrate 2-aminobenzoyl-L-arginyl-L-seryl-L-valyl-L-isoleucyl-L-(ϵ-2,4-dinitrophenyl)lysine. In this protocol, we significantly reduced enzyme concentration and introduced some minor modifications to decrease variation between replicates. The protocol was validated using emfourin, a novel proteinaceous metalloprotease inhibitor. Data obtained demonstrates that the developed assay method is an affordable approach for characterizing and screening various PLP inhibitors.
Graphical abstract:
Background
Protealysin-like proteases (PLPs) are a large group of zinc-containing metalloproteases belonging to the M4 family, according to the MEROPS database (www.ebi.ac.uk/merops) (Demidyuk et al., 2008, 2013). The interest in PLPs is primarily due to their possible involvement in bacterial pathogenesis, as they seem to be involved in bacterial invasion into eukaryotic cells, suppression of immune defense of some animals, and destruction of plant cell walls (Tsaplina et al., 2012, 2020; Demidyuk et al., 2013; Feng et al., 2014; Eshwar et al., 2018; Khaitlina et al., 2020).
We have previously developed a method for activity determination of protealysin (PLN), the prototype of PLP, from Serratia proteamaculans based on the hydrolysis of internally quenched fluorescent peptide substrate 2-aminobenzoyl-L-arginyl-L-seryl-L-valyl-L-isoleucyl-L-(ϵ-2,4-dinitrophenyl)lysine [Abz-RSVIK(Dnp)]. The proposed substrate was advantageous for quantitative activity analysis of PLN, as well as other M4 peptidases (Karaseva et al., 2019). Recently, we discovered emfourin (M4in) from S. proteamaculans, a novel potent slow-binding competitive proteinaceous inhibitor of PLN. To characterize the inhibition activity of M4in, we had to modify the PLN activity assay. Firstly, we significantly reduced the enzyme concentration and, in addition, introduced some minor modifications to decrease variation between replicates (Chukhontseva et al., 2021). Using the modernized protocol in the M4in study has shown that this approach is suitable for screening and characterizing PLP inhibitors.
Since Abz-RSVIK(Dnp) can be used to determine the activity of not only PLN but also other M4 family proteases (Karaseva et al., 2019), the proposed method can be adapted to determine the activity of inhibitors of various representatives of this family. Metalloproteases belonging to the M4 family are produced by significant human pathogens, such as Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Burkholderia cenocepacia, Enterococcus faecalis, Legionella pneumophila, Clostridium perfringens, and several pathogenic Vibrio species; their inhibitors, in the face of growing antibiotic resistance in bacteria, are considered as potential drug targets (Adekoya and Sylte, 2009). Thus, this method can be useful in the search for new antibacterial agents.
Materials and Reagents
Microcentrifuge tubes (1.5 mL) for reagent dilutions (SSI, catalog number: 1260)
Black 96-well polystyrene flat bottom microwell plate (Corning, catalog number: 3915)
0.20 μm membrane filter (Corning, catalog number: 431219)
Protealysin (PLN) was produced in E. coli and purified as described in Gromova et al. (2009). Although PLN was used in the development of the protocol, other M4 family proteases can also be used. In particular, we successfully implemented the protocol without any modification with thermolysin from Bacillus thermoproteolyticus (Serva, catalog number: 36015)
Emfourin (M4in) was produced in E. coli and purified as described in Chukhontseva et al. (2021). The activity of other proteinaceous or low-molecular-weight inhibitors of M4 family peptidases can also be determined using the protocol
Substrate: 2-aminobenzoyl-L-arginyl-L-seryl-L-valyl-L-isoleucyl-L-(ϵ-2,4-dinitrophenyl)lysine [Abz-RSVIK(Dnp)] (Peptide 2.0)
Tris(hydroxymethyl)aminomethane (Tris) (Sigma-Aldrich, catalog number: T1503)
Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) (Sigma-Aldrich, catalog number: T3253)
Dimethyl sulfoxide (DMSO) (MP Biomedicals, catalog number: 0219605525)
Assay buffer (see Recipes)
Substrate stock solution in DMSO (see Recipes). Can be stored at -20 °C without any loss in substrate properties; however, it is necessary to minimize freeze-thaw cycles
Protease (PLN) solution (see Recipes). Prepare immediately before analysis; do not store
Inhibitor (M4in) solution (see Recipes). Prepare immediately before analysis; do not store
Equipment
Desktop microcentrifuge (Eppendorf, Micro Centrifuge 5415)
Solid state laboratory thermostat for microcentrifuge tubes (DNA-technology, Thermit)
Temperature-controlled microplate reader capable of measuring fluorescence kinetics (BMG, CLARIOstar Plus)
Software
GraphPad Prism 8.0 (GraphPad Software)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Berdyshev, I. M., Karaseva, M. A. and Demidyuk, I. V. (2022). Assay for Protealysin-like Protease Inhibitor Activity . Bio-protocol 12(19): e4528. DOI: 10.21769/BioProtoc.4528.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 活性
药物发现 > 药物筛选
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link