- EN - English
- CN - 中文
Enhanced Ribonucleoprotein Immunoprecipitation (RIP) Technique for the Identification of mRNA Species in Ribonucleoprotein Complexes
用于鉴定核糖核蛋白复合物中 mRNA 种类的增强型核糖核蛋白免疫沉淀 (RIP) 技术
发布: 2022年10月05日第12卷第19期 DOI: 10.21769/BioProtoc.4526 浏览次数: 2854
评审: Chiara AmbrogioDavide BottaAnonymous reviewer(s)
Abstract
RNA binding proteins (RBPs) are critical regulators of cellular phenotypes, and dysregulated RBP expression is implicated in various diseases including cancer. A single RBP can bind to and regulate the expression of many RNA molecules via a variety of mechanisms, including translational suppression, prevention of RNA degradation, and alteration in subcellular localization. To elucidate the role of a specific RBP within a given cellular context, it is essential to first identify the group of RNA molecules to which it binds. This has traditionally been achieved using cross-linking-based assays in which cells are first exposed to agents that cross-link RBPs to nucleic acids and then lysed to extract and purify the RBP-nucleic acid complexes. The nucleic acids within the mixture are then released and analyzed via conventional means (e.g., microarray analysis, qRT-PCR, RNA sequencing, or Northern blot). While cross-linking-based ribonucleoprotein immunoprecipitation (RIP) has proven its utility within some contexts, it is technically challenging, inefficient, and suboptimal given the amount of time and resources (e.g., cells and antibodies) required. Additionally, these types of studies often require the use of over-expressed versions of proteins, which can introduce artifacts. Here, we describe a streamlined version of RIP that utilizes exclusion-based purification technologies. This approach requires significantly less starting material and resources compared to traditional RIP approaches, takes less time, which is tantamount given the labile nature of RNA, and can be used with endogenously expressed proteins. The method described here can be used to study RNA-protein interactions in a variety of cellular contexts.
Graphical abstract:
Background
Post-transcriptional regulation of gene expression enables rapid systematic cellular responses to various stimuli and stressors (Chua et al., 2020; Weskamp et al., 2021). Post-transcriptional regulation can be mediated by various cis- and trans-acting elements, including regulatory elements on the mRNA itself as well as microRNAs and RNA-binding proteins (RBPs) (Theil et al., 2018; Furlan et al., 2021; Ma et al., 2022). In cells, RBPs often exist within large complexes, known as ribonucleoprotein (RNP) complexes, that include the RBPs themselves alongside their mRNA binding partner(s) and other proteins/molecules. Through these interactions in RNP complexes, RBPs can regulate the expression, translation, and/or localization of the mRNA species to which they bind (Dvir et al., 2018; Formicola et al., 2019).
Importantly, a single RBP can bind to and regulate the expression of many mRNAs (Hentze et al., 2018). Thus, deregulation of RBP expression has wide-ranging consequences for cellular health and survival. Notably, deregulation of RBP activity has been implicated in numerous human pathologies, including neurological disorders, kidney disease, and various types of cancer (Chatterji and Rustgi, 2018; Wang et al., 2018; Shi et al., 2020; Kinoshita et al., 2021; Weskamp et al., 2021). In order to begin probing the mechanism via which deregulation of RBP expression leads to disease, it is essential to first identify the group of mRNA binding partners to which the RBP binds. By identifying a list of mRNA binding partners for a given RBP, researchers can begin identifying candidate mRNA partner(s) from the list with potential links to the observed phenotype(s) and can thus begin elucidating the mechanism via which deregulated RBP expression leads to a diseased state (Fakhraldeen et al., 2022).
Given the importance of identifying mRNA binding partners of RBPs, research efforts have focused on developing methodologies that can reproducibly purify and identify mRNAs from RNP complexes (Zhao et al., 2022). The most common methodologies developed thus far rely upon cross-linking of often over-expressed RBPs prior to immunoprecipitation (IP). Once the RNP complexes are purified via manual IP, the cross-links are reversed, and the RNA is extracted and then analyzed via the researchers’ preferred method (e.g., qRT-PCR, microarray analysis, Northern blot, or RNA-Seq). Multiple versions of this so-called ribonucleoprotein immunoprecipitation (RIP) procedure have been developed, including RIP-Chip (manual RIP followed by microarray analysis), CLIP (cross-linking and immunoprecipitation), iCLIP (individual nucleotide resolution CLIP), PAR-CLIP (photoactivatable ribonucleoside-enhanced CLIP), HiTS-RAP (high throughput sequencing–RNA affinity profiling), and various adaptations therefrom (Galgano and Gerber, 2011; Jain et al., 2011; Danan et al., 2016, 2022; Haecker and Renne, 2014; Spitzer et al., 2014; Guerreiro et al., 2014; Ozer et al., 2015; Sutandy et al., 2016; Diaz-Munoz et al., 2017; George et al., 2017; Perconti et al., 2019; Lewinski et al., 2020; Benhalevy and Hafner, 2020; Baldini and Labialle, 2021). These approaches to RIP have several drawbacks. First, introduction of exogenously expressed RBPs can affect the mRNA binding profile of the RBP. Second, the processes of cross-linking and reverse cross-linking themselves can introduce artifacts by inducing interactions that were previously absent/not physiologically relevant, or by not capturing all interactions. Third, performing the IP procedure manually is time consuming and may promote irreproducibility based on the handler. Finally, any added benefits of potentially being able to identify specific binding sites of RBPs with their mRNA binding partners using these techniques are overshadowed by the complex computational approaches required in order to perform such analyses (Jens, 2016; Huessler et al., 2019; Busch et al., 2020; Zhao et al., 2022).
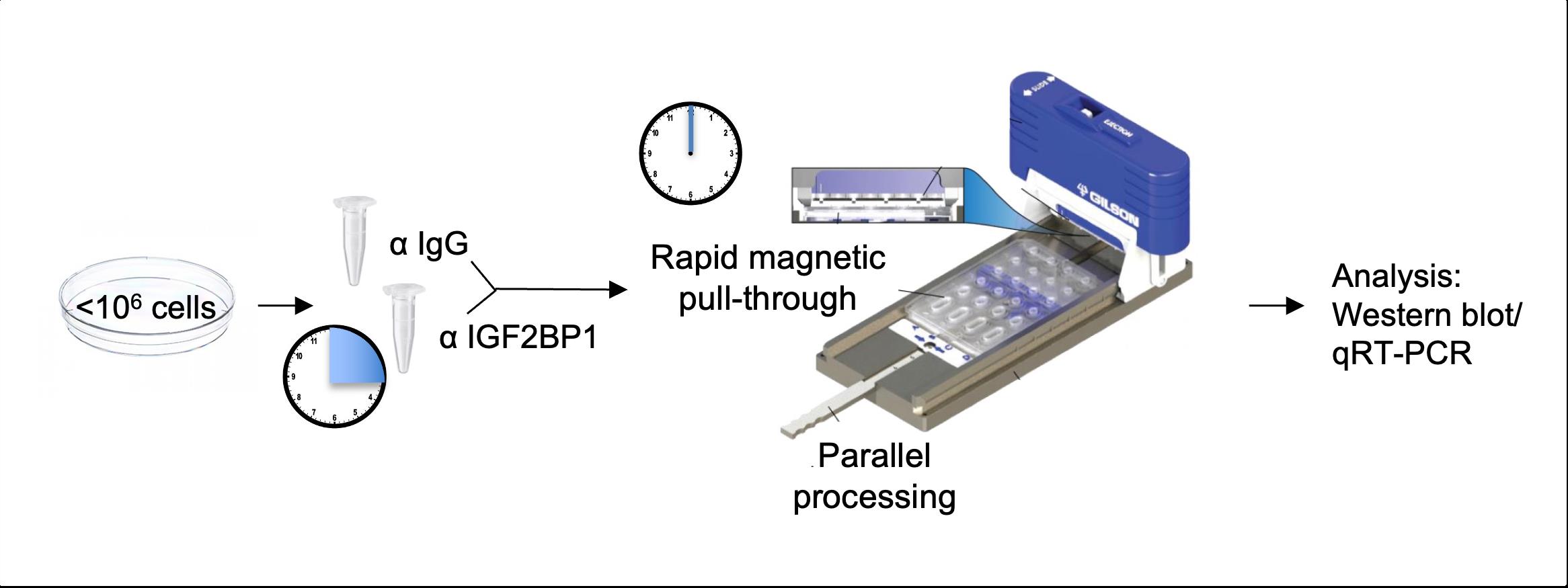
The method described here relies on exclusion-based sample preparation (ESP) technologies to perform RIP (Berry et al., 2015; Pezzi et al., 2018; Fakhraldeen et al., 2022). Specifically, we describe a procedure for performing RIP using the EXTRACTMAN device, which is an adaptation of the ESP-based Sliding Lid for Immobilized Droplet Extraction (SLIDE) technology. Not only does this method require less starting material, but it also offers significantly faster processing times, which is of particular relevance to RIP given the labile nature of RNA. Finally, the use of this sensitive method precludes the need to over-express the RBP of interest or to cross-link the lysate and instead allows for purification of the endogenous RBP in its native state. We describe the successful application of this method to identify mRNA binding partners of the RBP IGF2BP1 in the human embryonic kidney cell line HEK293T as well as in the human breast cancer cell lines MCF7 and MDA-MB-231 (Fakhraldeen et al., 2022). The extracted RNA was analyzed by qRT-PCR, but it can be analyzed using microarray analysis, RNA-Seq, or any endpoint of interest for RNA identification/profiling.
Materials and Reagents
293T human embryonic kidney cells (ATCC, catalog number: CRL-3216)
MCF7 cells (ATCC, catalog number: HTB-22)
MDA-MB-231 cells (ATCC, catalog number: CRM-HTB-26)
10 cm dishes (Fisherbrand, catalog number: FB0875712)
Cell scrapers (Fisherbrand, catalog number: 08-100-241)
DMEM, high glucose, HEPES (Thermo Fisher Scientific, Gibco, catalog number: 12430104)
DMEM, low glucose, pyruvate, HEPES (Thermo Fisher Scientific, Gibco, catalog number: 12320032)
Fetal bovine serum (FBS) [various vendors; pre-screened on mammary epithelial cells to identify lots that promoted cell growth without cell death (floating cells) over the course of four passages]
Penicillin/streptomycin (Thermo Fisher Scientific, Gibco, catalog number: 15070063)
1× phosphate buffered saline (PBS), pH 7.4 (Thermo Fisher Scientific, Gibco, catalog number: 10010023)
HEPES (1 M) (Gibco, Thermo Fisher Scientific, catalog number: 15630080)
MgCl2 (Merck/Sigma-Aldrich, catalog number: 208337)
KCl (Merck/Sigma-Aldrich, catalog number: P9541)
NonidetTM P 40 substitute (Fluka Biochemika, catalog number: 74385)
DTT (1,4-dithiothreitol; MilliporeSigma, catalog number: 111474)
RNaseOUTTM recombinant ribonuclease inhibitor (Thermo Fisher Scientific, Invitrogen, catalog number: 10777-019). Store at -20 °C and thaw on ice prior to use
Halt protease and phosphatase inhibitor single-use cocktail (Thermo Fisher Scientific, catalog number: 78442)
Dynabeads® Protein G (Novex by Life Technologies, catalog number: 10003D). Store at 4°C
Tween-20 (Merck/Sigma-Aldrich, catalog number: P9416)
RNeasy® mini kit (Qiagen, catalog number: 74104)
QuantiTect reverse transcription kit (Qiagen, catalog number: 205311)
Bradford reagent (Thermo Fisher Scientific, catalog number: 23238)
Transfer membrane Immobilon-P polyvinylidene difluoride (PVDF) (Merck/Millipore, catalog number: IPVH00010)
PreciseTM 4%–20% Tris-HEPES-SDS protein gels, 4.5 mm (Thermo Fisher Scientific, catalog number: 25244) or any pre-cast gel of choice
Anti-IMP-1 (D33A2) rabbit monoclonal primary antibody (Cell Signaling, catalog number: 8482)
Anti-vinculin mouse primary antibody, clone V284 (MilliporeSigma, catalog number: 05-386)
Anti-GAPDH (14C10) rabbit monoclonal primary antibody (Cell Signaling, catalog number: 2118)
Horseradish peroxidase (HRP) donkey anti-mouse polyclonal secondary antibody (Jackson ImmunoResearch, catalog number: 715-035-151)
HRP goat anti-rabbit polyclonal secondary antibody (Invitrogen, catalog number: G-21234)
HRP mouse anti-rabbit IgG (conformation-specific) (L27A9) secondary antibody (Cell Signaling, catalog number: 5127)
Non-immune rabbit IgG whole molecule control (Jackson ImmunoResearch, catalog number: 011-000-003)
Milk non-fat, dry milk powder (Kroger or equivalent)
Polysome lysis buffer (see Recipes)
RIP wash/elution buffer (see Recipes)
5% milk in TBS-Tween (see Recipes)
Equipment
EXTRACTMAN device (Gilson)
Spectrophotometer (Thermo Fisher Scientific, model: NanoDrop 2000)
Gel documentation system (Bio-Rad, Gel Doc XR+ Gel Documentation System)
Refrigerated centrifuge that can reach speeds of ≥12,000 rpm (must accommodate 50 mL tubes spun at 3,000 × g and 1.5 mL tubes spun at 12,000 rpm)
Plate centrifuge (Beckman, model: Allegra X15R)
Sonicator (Fisher Scientific 100)
Tube rotator that can hold 1.5 mL tubes; a standard rotisserie tube rotator with adaptors for Eppendorf tubes was used
qPCR machine (Applied Biosystems, ABI7900HT Fast Real-Time PCR System)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Fakhraldeen, S. A., Berry, S. M., Beebe, D. J. and Alexander, C. M. (2022). Enhanced Ribonucleoprotein Immunoprecipitation (RIP) Technique for the Identification of mRNA Species in Ribonucleoprotein Complexes. Bio-protocol 12(19): e4526. DOI: 10.21769/BioProtoc.4526.
- Fakhraldeen, S. A., Berry, S. M., Beebe, D. J., Roopra, A., Bisbach, C. M., Spiegelman, V. S., Niemi, N. M. and Alexander, C. M. (2022). Enhanced immunoprecipitation techniques for the identification of RNA-binding protein partners: IGF2BP1 interactions in mammary epithelial cells. J Biol Chem 298(3): 101649.
分类
细胞生物学 > 基于细胞的分析方法
癌症生物学 > 通用技术 > 生物化学试验 > 其它化合物
生物工程 > 生物医学工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link