- EN - English
- CN - 中文
Extraction and Quantification of Plant Hormones and RNA from Pea Axillary Buds
豌豆腋芽植物激素和 RNA 的提取与定量
发布: 2022年10月05日第12卷第19期 DOI: 10.21769/BioProtoc.4524 浏览次数: 2948
评审: Ansul LokdarshiAnonymous reviewer(s)

相关实验方案
用于研究真菌挥发性化合物(VCs)如何影响植物生长发育并通过SPME-GC-MS技术鉴定VCs的I板检测法
Wenzhao Wang [...] Seogchan Kang
2019年02月20日 8306 阅读
Abstract
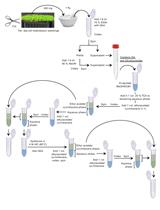
The quantification of plant hormones and related gene expression is essential to improve the understanding of the molecular regulation of plant growth and development. However, plant hormone quantification is still challenging due to extremely low endogenous levels and high chemical diversity. In this study, we present a convenient extraction protocol that enables the simultaneous extraction of both phytohormones and RNA from the same sample in a small quantity (approximately 10 mg). Using ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC–MS/MS), this protocol provides a method to quantify 13 phytohormones and their derivatives from four classes (cytokinin, auxin, abscisic acid, and gibberellin) at the speed of 14 min per sample.
Keywords: Phytohormone and RNA extraction (植物激素和RNA提取)Background
Phytohormones are endogenous signaling molecules that are involved in an immensely diverse range of plant physiological and developmental processes, which makes them critical for plant growth, development, and responses to biotic and abiotic stresses. Axillary bud outgrowth is a perfect example of a developmental process involving multiple phytohormones. Auxin, cytokinin, and strigolactone have been found to play major roles in triggering axillary bud dormancy (Barbier et al., 2019b). Abscisic acid and gibberellic acid are also involved in axillary bud outgrowth regulation (Yao and Finlayson, 2015; Charnikhova et al., 2017).
Despite their importance for plant growth regulation, not all phytohormones can yet be easily detected and quantified, which significantly limits the progression of phytohormone-related research (Cao et al., 2017; Liu et al., 2019). Due to various chemical classes and ultra-trace amounts of phytohormones in plant tissues, it is also difficult to measure a variety of phytohormone classes using a single separation method and analytical platform (Novák et al., 2017). Moreover, phytohormone levels vary between different plant tissues (Novák et al., 2017). Thus, it is important to select appropriate pre-treatment and quantification methods to boost the measurement sensitivity of targeted phytohormones (Yu et al., 2018). Ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC–MS/MS) has become the most efficient method for boosting measurement sensitivity, as it provides high selectivity and sensitivity for phytohormone profiling (Pan et al., 2010; Schäfer et al., 2016; Šimura et al., 2018). However, mass spectrometry sensitivity is strongly influenced by other compounds in plant materials, which suppress the ionization of target compounds (Trapp et al., 2014). Thus, a specific cleanup extraction method for phytohormones is needed. To quantify multiple phytohormone classes, many studies have used a time-consuming parallel extraction method for different classes of phytohormones (Cao et al., 2016; Xin et al., 2020). In addition to phytohormone profiling, monitoring gene expression is a key requirement for understanding the involvement of phytohormones in plant physiology and development (Šimura et al., 2018; Barbier et al., 2019b). However, a simultaneous extraction method for a wide range of phytohormones and RNA using one simple extraction method had not been previously reported.
Materials and Reagents
Phytohormone standards and internal standards (Table 1).
Table 1. Phytohormone standards and internal standards.
Reagent Supplier Classification Catalog number Storage temperature (°C) tZEATIN OlChemim STD 001 0301 -20 DHZ OlChemim STD 001 0601 -20 tZR OlChemim STD 001 0311 -20 DHZR OlChemim STD 001 0611 -20 tZMP OlChemim STD 001 5141 -20 iP OlChemim STD 001 0161 -20 iPR OlChemim STD 001 0171 -20 iPAMP OlChemim STD 001 5041 -20 IAA OlChemim STD 003 1531 -20 GA1 OlChemim STD 012 2491 -20 ABA OlChemim STD 013 2701 -20 D5-tZ OlChemim ISTD 030 0301 -20 D3-DZ OlChemim ISTD 030 0601 -20 d5-tZR OlChemim ISTD 030 0311 -20 d3-DZR OlChemim ISTD 030 0611 -20 d5-tZRP OlChemim ISTD 030 0311 -20 d6-iP OlChemim ISTD 030 0161 -20 d6-iPR OlChemim ISTD 030 0171 -20 d6-iPAMP OlChemim ISTD 030 5041 -20 d5-IAA OlChemim ISTD 031 1531 -20 d2-GA1* OlChemim ISTD 032 2491 -20 d2-GA20 OlChemim ISTD 032 2481 -20 d2-GA29 OlChemim ISTD 032 2471 -20 d6-ABA OlChemim ISTD 034 2721 -20 Tzeatin: trans-zeatin; DHZ: dihydrozeatin; tZR: trans-zeatin riboside; DHZR: dihydrozeatin ribodide; tZMP: trans-zeatin riboside-5'-monophosphate; iP: isopentenyladenine; iPR: isopentenyladenosine; iPAMP: isopentenyladenosine-5'-monophosphate; IAA: indole-3-acetic acid; ABA: abscisic acid; GA1, 20, and 29: gibberellin A1, A20, and A29; STD: phytohormone standard; ISTD: phytohormone internal standard.
*Can be replaced with d4-GA1 (product number: 032 2491) due to d2-GA1 not being commercially available anymore.
Acetonitrile (Merck, catalog number: 1.00030)
Methanol (Merck, catalog number: 1.06007)
Milli-Q water (Merk Milli-Q)
Acetic acid (Merck, catalog number: 5.33001)
Formic acid (Merck, catalog number: 5.33002)
Liquid nitrogen
Node 2 axillary buds from garden pea plants with five fully expanded leaves
Internal standard working solution (see Recipes)
Extraction solvent (see Recipes)
1% acetic acid (see Recipes)
Plant hormone standard solutions (see Recipes)
Equipment
2010 Geno Grinder (SPEX SamplePrep, model: 2010)
3 mm diameter 440C stainless steel balls for Geno/Grinder 2010 (SPEX SamplePrep, product number: 2151)
Refrigerated centrifuge (Eppendorf, model: 5425R)
Solid phase extraction manifold (Chromabond, model: 730150)
Rotational vacuum concentrator with cold trap (Christ, model: RVC 2-33)
Ultra-high pressure liquid chromatograph system (Shimadzu Corporation, model: Nexera X2)
Triple quadrupole linear ion trap mass spectrometry system (AB Sciex, model: 5500)
Laboratory scale (accuracy: 0.0001 g)
Sep-Pak tC18 cartridge (Waters, catalog number: WAT036820)
1.5 mL Eppendorf tube (Eppendorf, catalog number: 0030121872)
HPLC vial (Agilent, catalog number: 5188-6591)
HPLC cap (Agilent, catalog number: 5190-7024)
Kinetex C18 reversed phase UPLC column (2.1 mm ×100 mm, 1.7 μm) (Phenomenex, catalog number: 00A-4475-AN)
Vertex low-retention non-filtered pipette tip (SSIbio, catalog number: 4337N00)
15 mL Falcon tube (Corning, catalog number: 352096)
4 °C refrigerator and -20 °C and -80 °C freezer
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Cao, D., Barbier, F., Yoneyama, K. and Beveridge, C. A. (2022). Extraction and Quantification of Plant Hormones and RNA from Pea Axillary Buds. Bio-protocol 12(19): e4524. DOI: 10.21769/BioProtoc.4524.
分类
植物科学 > 植物生物化学 > 植物激素
生物化学 > 其它化合物 > 小分子
分子生物学 > RNA > qRT-PCR
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link