- EN - English
- CN - 中文
In vitro Fluorescence Imaging–based Actin Bundling Assay
基于体外荧光成像的肌动蛋白捆绑测定
发布: 2022年09月20日第12卷第18期 DOI: 10.21769/BioProtoc.4518 浏览次数: 2571
评审: David PaulSeham EbrahimMoriah R Beck
Abstract
Understanding the molecular and structural mechanisms that govern the assembly and organization of higher-order actin architecture requires the use of in vitro actin binding and bundling assays. Crosslinking of actin filaments into bundles can be monitored in vitro via several techniques, including negative staining/electron microscopy, low-speed co-sedimentation assay/SDS-PAGE, and fluorescence staining/confocal microscopy. We and others have previously characterized the N-BAR domain of ASAP1, an ADP-ribosylation factor GTPase-activating protein, as an actin-bundling module; we further identified key lysine residues responsible for actin cross-linking. Here, we use the ASAP1 BAR domain as an example and describe a detailed procedure for observing the actin bundle formation by confocal microscopy. This protocol requires small reaction volumes and takes advantage of bright commercially available fluorescent phalloidins, making it an ideal choice for medium-throughput screening of mutants or domain truncations in their ability to bundle actin.
Graphical abstract:
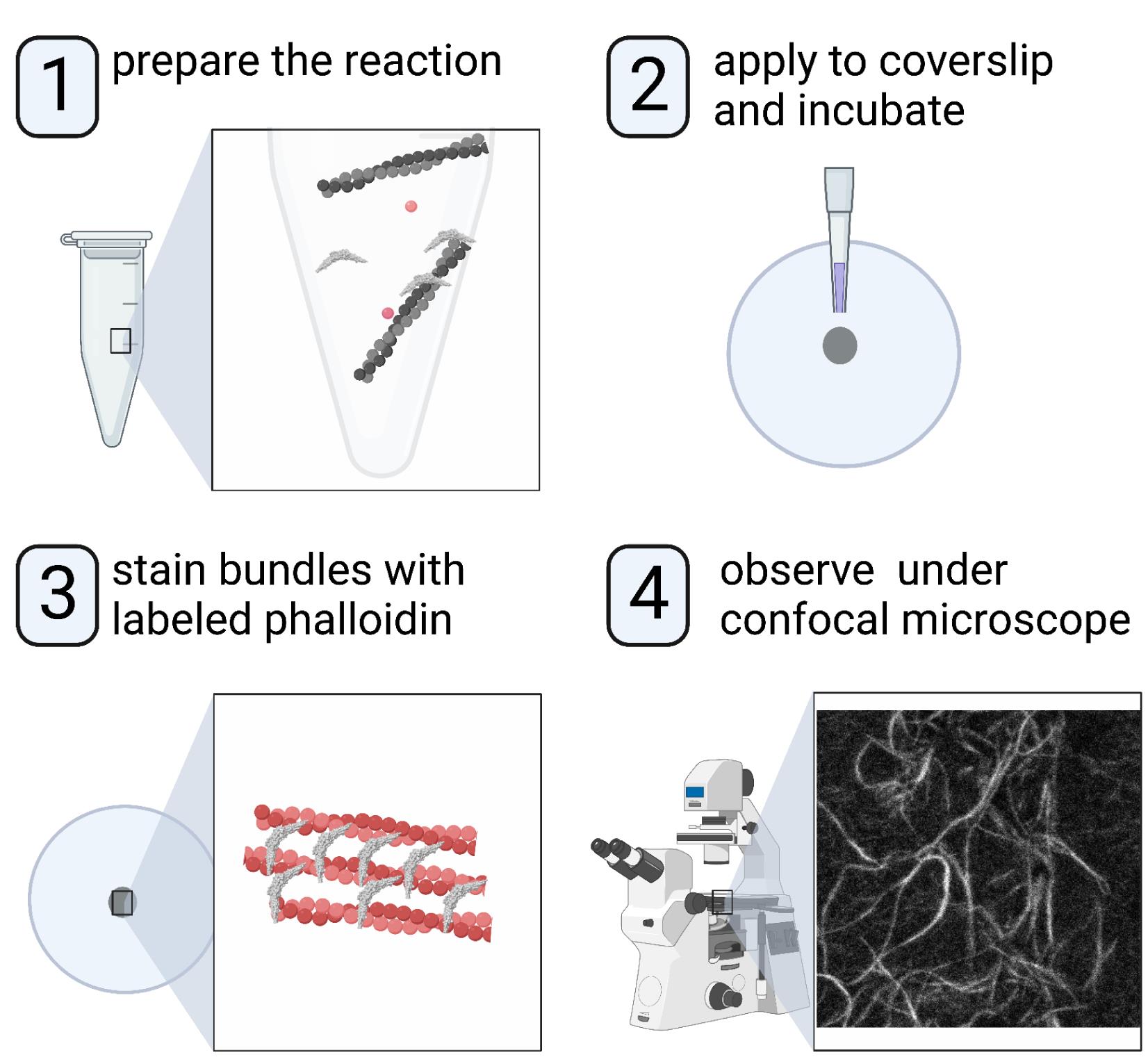
Background
Actin function in normal physiology and pathology depends on the assembly of actin filaments into higher-order structures. The regulation of higher-order actin structures is still being discovered. Advances in our understanding of these dynamics are facilitated by information-dense assays with quantifiable results. ASAP1 is an ADP-ribosylation factor GTPase-activating protein (GAP) that regulates the dynamics of filamentous actin–based structures, including stress fibers, focal adhesions, and circular dorsal ruffles (Randazzo et al., 2000; Oda et al., 2003; Bharti et al., 2007). We and others have previously found that the N-BAR domain of ASAP1 binds and bundles actin filaments and that its actin bundling activity is under auto-inhibitory regulation by its GAP and SH3 domains (Gasilina et al., 2019; Chen et al., 2020). We then set out to identify structural determinants on ASAP1 BAR domain that control actin bundling activity (Gasilina et al., 2022), which required careful quantification. Here, we detail a method to analyze the actin bundling activity of ASAP1 BAR-PH, which is applicable to other proteins with hypothesized actin-bundling activity. Unlike low-speed sedimentation assay, this method requires small quantities of actin filaments and test proteins and allows for simultaneous processing of several samples, which facilitates the evaluation of several concentrations, point mutants, domain truncations, or ionic strengths in a relatively short amount of time. In addition, because the filaments are directly visualized, information such as bundle width and length can be determined, which is not possible with the sedimentation assays; quantification is robust with methods that have been developed for analysis of images.
Materials and Reagents
Circular coverslips, German glass, 12 mm diameter, #1.5 thickness (Electron Microscopy Sciences, catalog number: 72291-02)
24-well flat bottom cell culture plate (Corning Costar, Corning, catalog number: 3526, or similar)
1.5–1.7 mL microcentrifuge tubes (Thomas Scientific, catalog number: 1138W14)
0.5 mL open-top thickwall polycarbonate tube (Beckman Coulter, catalog number: 343776)
50 mL conical tubes (Corning, catalog number: 352070)
0.22 µm syringe filters (Millex, Millipore-Sigma, catalog number: SLGPR33RS)
50 mL Luer tip syringes (BD Luer-Lok Tip, Fisher Scientific, catalog number: 14-820-11)
10 µL pipette tips, ends cut off to make a larger orifice
Frosted microscope slides (Fisherbrand, Fisher Scientific, catalog number: 12-550-343)
Ethanol/dry ice bath or liquid nitrogen for snap-freezing protein
Phosphate buffered saline (PBS) (Gibco, ThermoFisher Scientific, catalog number: 20012027)
Poly-L-lysine, 0.01% solution (Sigma-Aldrich, MilliporeSigma, catalog number: P4707-50ML)
Rabbit muscle G-actin, >99% pure (Cytoskeleton, Inc., catalog number: AKL99)
Note: Using actin prepared in-house from rabbit skeletal acetone powder is also fine, but care needs to be taken to verify that all bundling protein contaminants have been removed.
ASAP1 N-BAR recombinant protein, expressed and purified as described previously (Gasilina et al., 2019, 2022)
α-actinin (Cytoskeleton, Inc., catalog number: AT01)
Note: It is highly recommended to use a bona fide actin bundling protein, such as α-actinin or vinculin tail, as a positive control.
Bovine serum albumin (BSA) (Sigma-Aldrich, Millipore Sigma, catalog number: B8667). Aliquot into 100 µL volumes and snap freeze. Store at -80 °C.
Methanol ≥99.9% (J.T. Baker, VWR International, catalog number: 9093-02)
Fluorescently labeled phalloidin [rhodamine phalloidin (Invitrogen, ThermoFisher Scientific, catalog number: R415), or Alexa Fluor 488 phalloidin (Invitrogen, ThermoFisher Scientific, catalog number: A12379)]. Reconstitute in 1.5 mL of 100% methanol and store at -20 °C.
Note: Although any bright and photostable fluorescent phalloidin conjugate can be used to stain and visualize bundles, the use of fluorophores in the visible spectra will facilitate finding and focusing on the sample during data acquisition.
Adenosine 5’-triphosphate disodium salt (ATP) (Cytoskeleton, Inc., catalog number: BSA04). Reconstitute with 1 mL of cold 100 mM Tris, pH 7.5 (Recipe 7), for a 100 mM stock, aliquot into 5–100 µL portions and 10–50 µL portions, snap freeze, and store at -80 °C. Note that the ATP from cytoskeleton is lyophilized from a solution buffered to pH 7, and, consequently, the solutions do not require additional adjusting of the pH.
Paraformaldehyde, 16%, methanol-free (Electron Microscopy Sciences, catalog number: 15710)
Dako fluorescence mounting medium (Agilent, catalog number: S302380-2)
Calcium chloride (CaCl2) (Sigma-Aldrich, Millipore Sigma, catalog number: 223506)
Potassium chloride (KCl) (Sigma-Aldrich, Millipore Sigma, catalog number: P3911)
Magnesium chloride (MgCl2) (Sigma-Aldrich, Millipore Sigma, catalog number: M9272)
Tris-HCl, 1 M, pH 7.5 (KD Medical, catalog number: RGF-3350)
Dithiothreitol (DTT) Cleland’s reagent (MP Biomedicals, VWR International, catalog number: 0219482101)
1× G-actin buffer (see Recipes)
10× actin polymerization buffer (see Recipes)
1× F-actin buffer (see Recipes)
1 M CaCl2 (see Recipes)
1 M KCl (see Recipes)
1 M MgCl2 (see Recipes)
Tris-HCl, 1 M, pH 7.5 (see Recipes)
1 M DTT (see Recipes)
Equipment
P10, P20, and P200 micropipettes
Benchtop microcentrifuge for 1.5/2 mL tubes
Small volume ultra-centrifuge (e.g., Thermo Scientific Sorvall MTX with S120-AT3 rotor)
Confocal microscope equipped with oil-immersion objective, preferably with a tile scanning option (we use a 63× objective, but given the size of the bundles, any objective between 40× and 100× is suitable.)
Software
Fiji/ImageJ (National Institutes of Health, imagej.nih.gov)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gasilina, A. and Randazzo, P. A. (2022). In vitro Fluorescence Imaging–based Actin Bundling Assay. Bio-protocol 12(18): e4518. DOI: 10.21769/BioProtoc.4518.
- Gasilina, A., Yoon, H. Y., Jian, X., Luo, R. and Randazzo, P. A. (2022). A lysine-rich cluster in the N-BAR domain of ARF GTPase-activating protein ASAP1 is necessary for binding and bundling actin filaments. J Biol Chem 298(3): 101700.
分类
生物化学 > 蛋白质 > 成像
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link