- EN - English
- CN - 中文
Human Skin Explant Preparation and Culture
人体皮肤外植体制备和培养
发布: 2022年09月20日第12卷第18期 DOI: 10.21769/BioProtoc.4514 浏览次数: 3921
评审: Giusy TornilloDongsheng JiangGiovanna Piovani
Abstract
The ex vivo experimentation with surgically discarded human skin represents a unique methodology amenable for mechanism and pharmacologic agent studies without the involvement of human subjects. Here, we describe a protocol that includes preparation, culture, and stimulation of human skin explants, and subsequent analyses by quantitative reverse transcription PCR and immunostaining. This protocol may also be applied for ex vivo studies of murine skin, reducing animal numbers and potentially harmful treatments. In our hands, this protocol has been used for wound healing, viral infection, and hair growth–related studies.
Graphical abstract:
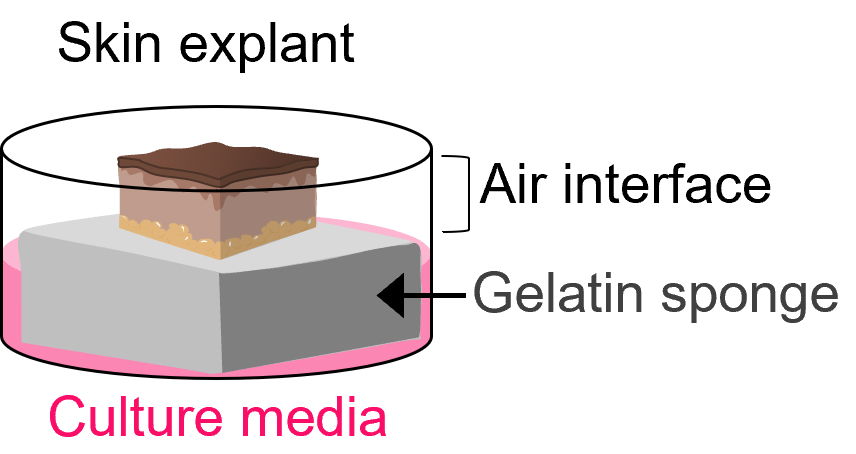
Cartoon of explant skin culture. Skin explant sits on top of a gelatin surgical sponge saturated with culture medium at an air–liquid interface.
Background
Genetic animal models are powerful in recapitulating complex tissue environment with intact circulation. Mice are by far the most frequently used animal for gene function, disease modeling, and pharmacological studies. However, murine skin and human skin are architecturally different and show differences in their responses to injury (Zomer and Trentin, 2018). It is not surprising that findings using murine skin often need corroboration in human skin models that harbor tissue-resident immune cells (Zomer and Trentin, 2018).
Significant technical advances have been achieved in epidermal keratinocyte culture in vitro, allowing procurement and expansion of primary keratinocytes. However, monolayer cell culture does not always recapitulate in vivo processes and often lacks tissue-resident immune cells. The organotypic cell culture model generated with primary or immortalized keratinocytes allows three-dimensional epidermal stratification and incorporation of certain dermal cells such as fibroblasts (Smits et al., 2017). Each of these methods has limitations in recapitulating skin biology.
Our lab has adapted a protocol for using surgically discarded human skin samples for ex vivo studies (Shannon et al., 2022), such as wound assays, cytokine stimulation, and viral infections. The protocol described below includes necessary materials, reagents, equipment, and procedures.
Materials and Reagents
1.7 mL microtube (Genesee Scientific, catalog number: 22-281LR)
SURGIFOAM® absorbable gelatin sponge (Ethicon, catalog number: 1969)
12-well cell culture plates, flat bottom wells, TC-treated (GenClone 25-106)
Sterile 1× phosphate buffered saline (PBS) (Gibco, catalog number: 14190144)
Penicillin–streptomycin–glutamine (100×) (Gibco, catalog number: 10378016)
EpiLifeTM CF kit (Gibco, catalog number: MEPICF500)
Note: This kit contains 500 mL of EpiLifeTM calcium-free media and 500 μL of 0.06 M CaCl2 (see Recipes).
Human keratinocyte growth supplement (HKGS, 100×) (Gibco, catalog number: S0015)
Adwin scientific Tissue-Tek Cryomold, intermediate (Fisher Scientific, catalog number: NC9511236)
Tissue-Tek optimum cutting temperature (OCT) compound (VWR, catalog number: 25608-930)
Rat anti-human Integrin α6 CD49f antibody (BioLegend, catalog number: 313602, diluted 1:500)
Alexa Fluor 647-conjugated goat anti-rat secondary antibody (BioLegend, catalog number: 313609). Diluted 1:400
Prolong Gold antifade reagent (ThermoFisher Scientific, catalog number: P36930)
TRIzolTM reagent (ThermoFisher Scientific, catalog number: 15596026)
Hoechst 33342, trihydrochloride, trihydrate (ThermoFisher Scientific, catalog number: H3570)
iScripTM cDNA synthesis kit (Bio-Rad, catalog number: 1708891)
Culture medium (see Recipes)
Wash buffer (see Recipes)
70% ethanol (see Recipes)
Equipment
CO2 incubator (Panasonic, model: MCO-170AICUVL-PA)
Technocut® disposable scalpels, sterile, MYCO Medical, No. 20 (VWR, catalog number: 10148-894)
Forceps (VWR, catalog number: 89259-946)
Surgical scissors (VWR, catalog number: 76192-134)
Pipettes (ThermoFisher Scientific, model: FinnpipetteTM F2 variable volume pipettes, catalog number: 4701070)
Olympus IX73 fluorescent microscope (Olympus Corporation Microscopy Technologies)
Nanodrop 1000
-80 °C freezer
Leica Cryocut 1800
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Shannon, J. L., Kirchner, S. J. and Zhang, J. Y. (2022). Human Skin Explant Preparation and Culture. Bio-protocol 12(18): e4514. DOI: 10.21769/BioProtoc.4514.
分类
细胞生物学 > 组织分析 > 组织培养
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link