- EN - English
- CN - 中文
Protein Tyrosine Phosphatase Biochemical Inhibition Assays
蛋白酪氨酸磷酸酶生化抑制测定
发布: 2022年09月20日第12卷第18期 DOI: 10.21769/BioProtoc.4510 浏览次数: 3022
评审: Chiara AmbrogioZheng Zachory WeiKarem A CourtAnonymous reviewer(s)
Abstract
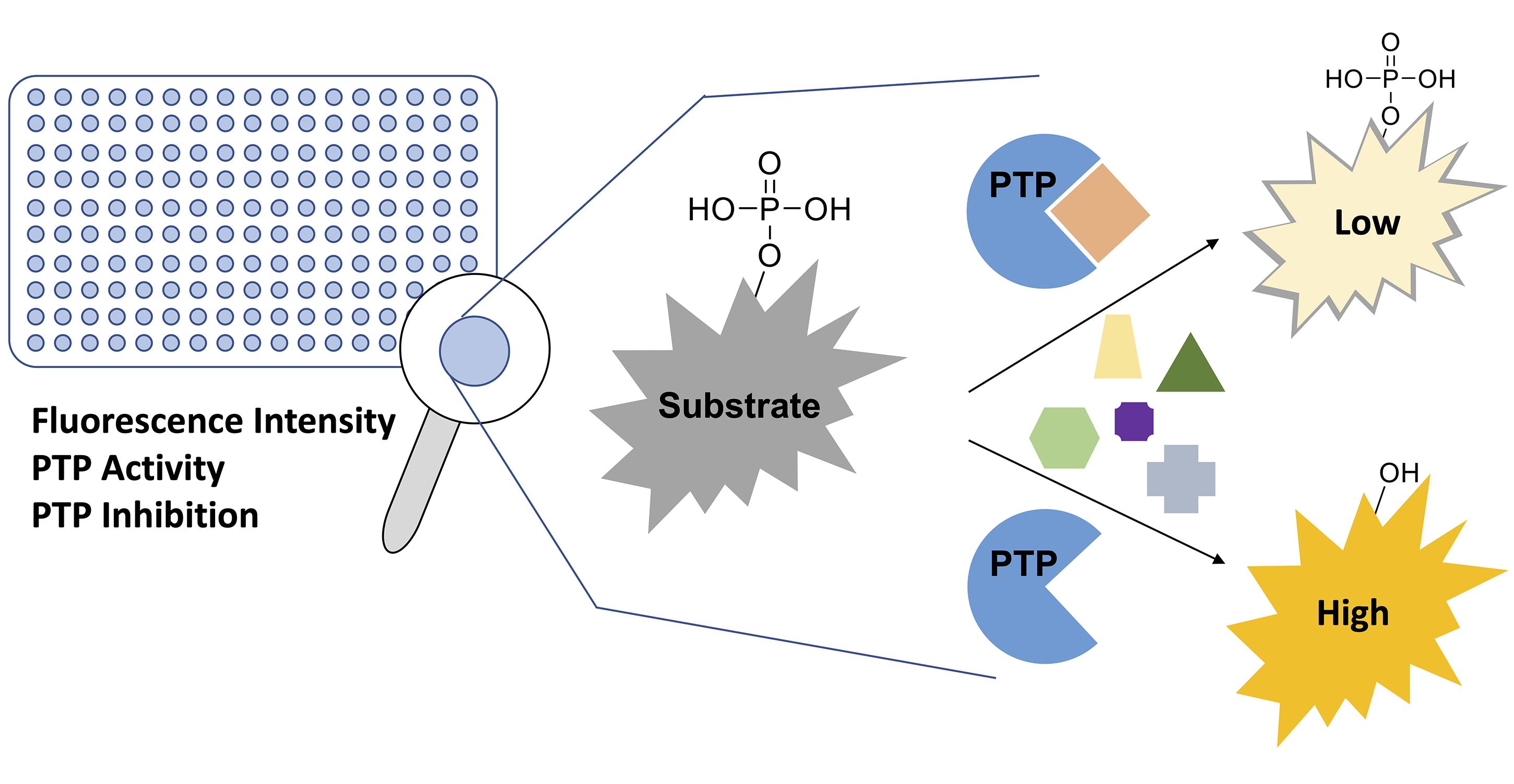
Disturbance of the dynamic balance between protein tyrosine phosphorylation and dephosphorylation, modulated by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), is known to be crucial for the development of many human diseases. The discovery of agents that restore this balance has been the subject of many drug research efforts, most of which have focused on tyrosine kinase inhibitors (TKIs), resulting in the development of more than 50 FDA-approved TKIs during the past two decades. More recently, accumulating evidence has suggested that members of the PTP superfamily are also promising drug targets, and efforts to discover tyrosine phosphatase inhibitors (TPIs) have increased dramatically. Here, we provide protocols for determining the potency of TPIs in vitro. We focus on the use of fluorescence-based substrates, which exhibit a dramatic increase in fluorescence emission when dephosphorylated by the PTP, and thus allow setting up highly sensitive and miniaturized phosphatase activity assays using 384-well or 1536-well microplates and a continuous (kinetic) assay format. The protocols cover PTP specific activity assays, Michaelis–Menten kinetics, dose-response inhibition assays, and dose-response data analysis for determining IC50 values. Potential pitfalls are also discussed. While advanced instrumentation is utilized for compound spotting and liquid dispensing, all the assays can be adapted to existing equipment in most laboratories. Assays are described for selected PTP drug targets, including SHP2 (PTPN11), PTP1B (PTPN1), STEP (PTPN5), and VHR (DUSP3). However, all protocols are applicable to members of the PTP enzyme family in general.
Graphical abstract:
Background
Protein tyrosine phosphorylation is a reversible posttranslational modification (PTM) and a fundamentally important mechanism in eukaryotic cell signal transduction and regulation (Hunter, 2009). Perturbations in tyrosine phosphorylation can lead to the development of many human diseases, including cancer, neurological disorders, autoimmunity, and immunodeficiency, as well as cardiovascular, metabolic, and infectious diseases (Tautz et al., 2006; Vang et al., 2008; Labbe et al., 2012; Goebel-Goody et al., 2012; Zhang, Z. Y. et al., 2015; Menegatti, 2022). Targeting protein tyrosine kinases (PTKs) has been a major focus of drug discovery efforts in the last two decades, resulting in more than 50 FDA-approved tyrosine kinase inhibitors (TKIs) (Cohen et al., 2021). On the other hand, the discovery of clinical candidates that target protein tyrosine phosphatases (PTPs) has significantly lagged behind the kinases for multiple reasons (reviewed in Tautz et al., 2013; Tonks, 2013; Stanford and Bottini, 2017). Unquestionably, an inflection point in tyrosine phosphatase inhibitor (TPI) research was the discovery of SHP099, the first truly selective and drug-like inhibitor of the SHP2 (PTPN11) phosphatase (Chen et al., 2016). The compound has since served as a blueprint for several investigational drugs that are currently being tested in phase 1/2 clinical trials for the treatment of solid tumors (Song et al., 2022).
The success in bringing SHP2 inhibitors into the clinic has garnered a new wave of interest in targeting PTPs. Here, we provide protocols for determining the potency of TPIs in enzymatic phosphatase assays. The experiments described cover: 1) PTP activity assays, to determine a suitable enzyme concentration; 2) Michaelis–Menten kinetics, to determine the Michaelis–Menten constant (Km) of the substrate for a specific PTP; and 3) dose-response inhibition assays and dose-response data analysis, to determine IC50 values of potential inhibitors. We utilized advanced instrumentation for automated compound spotting and liquid dispensing. However, the assays described herein can be adapted to existing equipment in most laboratories. While our protocols are applicable to PTPs in general, we show examples that utilize four specific phosphatases with promising therapeutic potential in various diseases, including cancer [SHP2, PTP1B (Vainonen et al., 2021)], type II diabetes [PTP1B (Zhang, Z. Y. et al., 2015)], Alzheimer’s disease [STEP (Lambert et al., 2021)], as well as arterial thrombosis, sepsis, and septic shock [VHR (Tautz et al., 2015; Singh et al., 2015)].
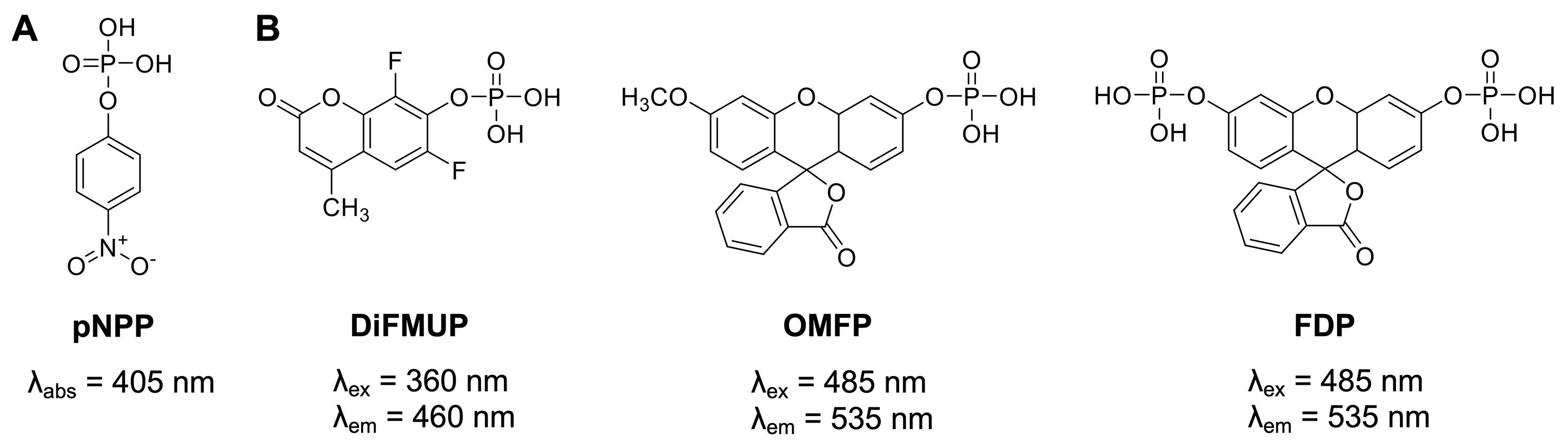
Figure 1. Generic protein phosphatase substrates used for PTP enzymatic assays. (A) The colorimetric substrate p-nitrophenyl phosphate (pNPP). (B) The fluorogenic substrates 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP), 3-O-methylfluorescein phosphate (OMFP), and fluorescein diphosphate (FDP).
Historically, a widely used generic protein phosphatase substrate is p-nitrophenyl phosphate (pNPP) (Bessey et al., 1946) (Figure 1A). Conversion of pNPP generates p-nitrophenol, which can be directly monitored via its absorbance at 405 nm. While the absorbance of p-nitrophenol is linear over a relatively wide range of concentrations (approximately 5–500 μM), colored small molecules of interest can absorb light at similar frequencies, resulting in potential false negative results. Moreover, relatively high concentrations of recombinant PTPs (in the mid nanomolar range) are typically required to produce sufficient p-nitrophenol signal to background (S/B) and signal to noise (S/N) ratios. More recently, fluorogenic protein phosphatase substrates such as 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP), 3-O-methylfluorescein phosphate (OMFP), or fluorescein diphosphate (FDP) (Figure 1B) have been utilized for PTP fluorescence intensity assays (Huang et al., 1999; Welte et al., 2005; Tierno et al., 2007; Tautz and Sergienko, 2013). These substrates have a low fluorescence in the phosphorylated state but become strong fluorophores when dephosphorylated. Typically, PTP assays using fluorogenic substrates are several orders of magnitude more sensitive than comparable pNPP assays, thus requiring significantly less recombinant PTP enzyme (picomolar to low nanomolar concentrations). Additionally, the fluorescence emission of the dephosphorylated products can be measured over a wide range of concentrations (approximately 10 nM to >100 μM) with excellent S/B and S/N ratios for highly reproducible PTP assays in continuous (kinetic) mode, which allows for the most accurate determination of the initial velocity rates (V). PTP assays using fluorogenic substrates can be easily miniaturized and performed in 384-well or 1536-well formats, allowing for efficient dose-response testing of candidate inhibitors. For the protocols provided here, we employ DiFMUP and/or OMFP with standard volume 384-well microplates. These plates do not require automated liquid handling and are amenable to manual liquid transfers using multichannel pipettes. However, laboratories with advanced equipment will experience no difficulty in adapting the protocols to a 1536-well format, as we have successfully performed similar assays using 1536-well microplates in a total assay volume as small as 5 μL.
Notes and Considerations
PTPs typically are most active at a pH between 5.5 and 6 (Groen et al., 2005). Our standard assay buffer that works well for most PTPs is Bis-Tris used at pH 6 (see Recipe 1). Buffer systems closer to physiological pH (e.g., Tris buffer at pH 7.4) may be used as well. For inhibition assays, we recommend not using buffers containing sulfonic acids such as HEPES, which can compete with inhibitor binding at the active site. A reducing agent such as dithiothreitol (DTT) ensures that the PTP catalytic cysteine is in the active, reduced state. It also prevents potentially oxidizing compounds from nonspecifically inhibiting the PTP through oxidation of the catalytic Cys. The addition of a detergent such as 0.01% Tween 20 is highly recommended, as it stabilizes the protein over the course of the assay and reduces the likelihood of promiscuous, aggregate-based inhibition (Feng and Shoichet, 2006). Bovine serum albumin (BSA) or globulin proteins may be used as detergent substitutes. For a detailed description of buffer optimization experiments, we refer to our previous publication (Tautz and Sergienko, 2013).
Fluorogenic substrates such as DiFMUP or OMFP may encounter compound spectral interference, either via compound autofluorescence or compound-induced fluorescence quenching. In our experience, the DiFMUP assay, which relies on the near-UV/blue spectral range (λex = 360 nm, λem = 460 nm), is more prone to such interference than the red-shifted OMFP assay (λex = 485 nm, λem = 535 nm). A pre-read of the assay plate (containing enzyme solution and compound) before addition of the substrate will typically show any potential compound autofluorescence. Likewise, IC50 curves going beyond 100% inhibition may indicate fluorescence quenching. When compound fluorescence interference is suspected, an increase in fluorescent product by using either greater enzyme concentrations or longer reaction times, while still staying within the linear range of substrate conversion, could lessen such interference effects. However, the best approach to mitigate such issues is to retest the suspected compounds using orthogonal substrates (e.g., using OMFP and/or pNPP instead of DiFMUP).
In our protocols, we use acoustic droplet dispensing of compound DMSO stock solutions, allowing for the transfer of nanoliter quantities. Specifically, when we use 384-well standard volume plates and a total assay volume of 25 μL, we transfer 250 nL of compound DMSO stock solution, which results in a final DMSO concentration of 1% that is typically well tolerated by recombinant PTPs. For manual transfer of compound stock solutions using a pipette, we recommend transferring no less than 1 μL to ensure accuracy. In our experience, when using a 1 μL transfer, the corresponding final DMSO concentration of 4% has no considerable effect on PTP stability or activity. In any case, the DMSO content should be kept at an equal amount in all wells, including wells for positive and negative controls.
Whenever feasible, we prefer using OMFP over DiFMUP because of the OMFP red-shifted excitation and emission wavelengths compared to DiFMUP, which lower the chances of compound fluorescence interference. However, one potential issue is the limited aqueous solubility of OMFP that requires initial dissolution in DMSO, resulting in extra DMSO added to the reaction mixtures. Using a 10 mM OMFP stock solution in DMSO (which reaches the limit of OMFP solubility in DMSO), the final DMSO concentration is usually manageable for most PTPs, for which OMFP Km values are in the mid- to low-micromolar range. However, for some PTPs (e.g., SHP2), the OMFP Km is in the high micromolar range, which makes it difficult (or impossible) to keep the final DMSO concentration at the recommended ≤5%, which ensures negligible impact on the stability and activity of the recombinant PTP.
Materials and Reagents
384-well black, flat bottom, standard volume microplates (Greiner Bio-One FLUOTRAC 200, catalog number: 781076)
Aluminum adhesive plate seals (Sigma-Aldrich, catalog number: Z721557)
1.5 mL Eppendorf tubes
15 mL conical tubes
50 mL conical tubes
Recombinant human PTPs with a purity of at least 95% according to SDS-PAGE gel electrophoresis: full-length SHP2 wild-type (SHP2-WT), SHP2 catalytic domain (SHP2cat, aa 237–529), PTP1B (aa 1–300), full-length STEP46, and full-length VHR.
Dually phosphorylated IRS-1 peptide (synthesized by PepMic, Suzhou, China) for activating SHP2-WT
Bis-Tris (Research Product International, catalog number: B75000)
Sodium chloride (NaCl, Sigma-Aldrich, catalog number: S3014)
EDTA tetrasodium salt dihydrate (BioWorld, catalog number: 40500024)
3-O-methylfluorescein phosphate cyclohexylammonium salt (OMFP; in-house synthesis)
6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP; ThermoFisher Scientific, catalog number: D22065)
Tween 20 (Fisher Bioreagents, catalog number: BP337)
DL-dithiothreitol (DTT; BioWorld, catalog number: 40400120)
Dimethyl sulfoxide (DMSO; Sigma-Aldrich, catalog number: D8418)
Milli-Q water: water purified using a Millipore Milli-Q lab water system
Bis-Tris buffer (see Recipes)
10 mM OMFP stock solution in DMSO (see Recipes)
10 mM DiFMUP stock solution in water (see Recipes)
1 M DTT stock solution (see Recipes)
Equipment
Fluorescence microplate reader with filters for 360 nm, 460 nm, 485 nm, and 535 nm (Tecan, Spark Multimode Microplate Reader)
Tabletop centrifuge with a swinging-bucket rotor (Eppendorf, 5810R equipped with A-4-62 rotor and MTP bucket)
MultidropTM Combi reagent dispenser (Thermo Fisher Scientific, catalog number: 5840300)
Small tube dispensing cassette (Thermo Fisher Scientific, catalog number: 24073290 or 24073295)
E1-ClipTipTM multichannel pipettes (Thermo Fisher Scientific, various volumes)
Echo® 555 Liquid Handler (Labcyte)
Note: To spin down plates, the Eppendorf tabletop centrifuge was used at 1,000 rpm (approximately 172 × g).
Software
Chemical and Biological Information Systems (CBIS, ChemInnovation Software, Inc.)
GraphPad PrismTM v.9 (GraphPad Software, LLC.)
MagellanTM data analysis software (Tecan)
Microsoft® Excel (Microsoft)
Note: Slopes from kinetic progression curves can be calculated using either Magellan, GraphPad Prism, or Excel. Michaelis–Menten parameters can be calculated in GraphPad Prism (or equivalent programs). Both GraphPad Prism and CBIS allow for straightforward analysis of dose-response data and calculation of IC50 values. CBIS has the advantage of providing a more automated and convenient environment for analyzing larger data sets.
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Baranowski, M. R., Wu, J., Han, Y. N., Lambert, L. J., Cosford, N. D. P. and Tautz, L. (2022). Protein Tyrosine Phosphatase Biochemical Inhibition Assays. Bio-protocol 12(18): e4510. DOI: 10.21769/BioProtoc.4510.
- Raveendra-Panickar, D., Finlay, D., Layng, F. I., Lambert, L. J., Celeridad, M., Zhao, M., Barbosa, K., De Backer, L. J. S., Kwong, E., Gosalia, P., et al. (2022). Discovery of novel furanylbenzamide inhibitors that target oncogenic tyrosine phosphatase SHP2 in leukemia cells. J Biol Chem 298(1): 101477.
分类
生物化学 > 蛋白质 > 活性
药物发现 > 药物筛选
药物发现 > 药物设计
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link