- EN - English
- CN - 中文
A Semi-quantitative Scoring System for Green Histopathological Evaluation of Large Animal Models of Acute Lung Injury
用于急性肺损伤大型动物模型绿色组织病理学评价的半定量评分系统
发布: 2022年08月20日第12卷第16期 DOI: 10.21769/BioProtoc.4493 浏览次数: 4635
评审: Meenal SinhaJohnatas Dutra SilvaJulie WeidnerJennifer L. Larson-Casey
Abstract
Acute respiratory distress syndrome (ARDS) is a life-threatening, high mortality pulmonary condition characterized by acute lung injury (ALI) resulting in diffuse alveolar damage. Despite progress regarding the understanding of ARDS pathophysiology, there are presently no effective pharmacotherapies. Due to the complexity and multiorgan involvement typically associated with ARDS, animal models remain the most commonly used research tool for investigating potential new therapies. Experimental models of ALI/ARDS use different methods of injury to acutely induce lung damage in both small and large animals. These models have historically played an important role in the development of new clinical interventions, such as fluid therapy and the use of supportive mechanical ventilation (MV). However, failures in recent clinical trials have highlighted the potential inadequacy of small animal models due to major anatomical and physiological differences, as well as technical challenges associated with the use of clinical co-interventions [e.g., MV and extracorporeal membrane oxygenation (ECMO)]. Thus, there is a need for larger animal models of ALI/ARDS, to allow the incorporation of clinically relevant measurements and co-interventions, hopefully leading to improved rates of clinical translation. However, one of the main challenges in using large animal models of preclinical research is that fewer species-specific experimental tools and metrics are available for evaluating the extent of lung injury, as compared to rodent models. One of the most relevant indicators of ALI in all animal models is evidence of histological tissue damage, and while histological scoring systems exist for small animal models, these cannot frequently be readily applied to large animal models. Histological injury in these models differs due to the type and severity of the injury being modeled. Additionally, the incorporation of other clinical support devices such as MV and ECMO in large animal models can lead to further lung damage and appearance of features absent in the small animal models. Therefore, semi-quantitative histological scoring systems designed to evaluate tissue-level injury in large animal models of ALI/ARDS are needed. Here we describe a semi-quantitative scoring system to evaluate histological injury using a previously established porcine model of ALI via intratracheal and intravascular lipopolysaccharide (LPS) administration. Additionally, and owing to the higher number of samples generated from large animal models, we worked to implement a more sustainable and greener histopathological workflow throughout the entire process.
Keywords: Green histology (绿色组织学)Background
Acute respiratory distress syndrome (ARDS) is a highly heterogeneous and life-threatening disease with reported mortality rates ranging from 30%–50% worldwide (Gonzales et al., 2015; Maca et al., 2017; Potere et al., 2020). Caused by a variety of infectious and non-infectious injuries (e.g., trauma, surgery, burn wounds, gastric aspiration, or inhalation of highly toxic substances), ARDS is characterized by diffuse alveolar damage that causes a rapid decline in lung function (Matthay et al., 2019). Infectious causes of ARDS may be direct lung injury through different pathogens, including viral (e.g., SARS-CoV-2), bacterial (e.g., Streptococcus pneumoniae), and fungal colonization (e.g., aspergillosis in mechanically ventilated patients). Additionally, ARDS also commonly results from indirect lung injury through systemic infection (e.g., urinary tract and soft tissue or skin infections), fragments of pathogens [e.g., lipopolysaccharide (LPS)], and inflammatory cytokines in sepsis patients (Lee, 2017).
The clinical diagnosis of ARDS uses the Berlin criteria, which consists of four main characteristics (ARDS Definition Task Force et al., 2012; Ferguson et al., 2012): 1) timing within one week of known injury or new/worsening respiratory symptoms; 2) presence of bilateral opacities consistent with pulmonary edema visible on chest imaging (X-ray or CT scan), which are not fully justified by lobar/lung collapse or nodules; 3) respiratory failure not fully justified by heart failure or fluid overload (hydrostatic edema has to be excluded); and 4) hypoxemia [partial pressure of arterial oxygen/fraction of inspired oxygen (PaO2/FiO2) ≤ 300mm Hg with positive end expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) ≥ 5 cm H2O]. Compromised oxygenation levels can be further divided into three levels: Mild, Moderate, and Severe. Despite this improved classification system, ARDS remains a disease with high clinical heterogeneity, with no effective pharmacologic treatments.
While in vitro models recapitulating some features of ARDS exist, the use of both small and large animal models remains the main experimental tool for preclinical research. Several different models have been used to mimic the onset and development of acute lung injury (ALI), with the major factor differentiating them being the manner or source of injury. Different agents, such as oleic acid, lipopolysaccharide (LPS), gastric contents (or hydrochloric acid directly), hyperoxia, and bacterial or viral administration have all been shown to be capable of inducing acute lung injury as well as the use of high tidal volumes and low PEEP to induce ventilator induced lung injury (Ballard-Croft et al., 2012; Matute-Bello et al., 2008; Arora et al., 2019; Tiba et al., 2021). The choice of injury method and species used depends on the features to be investigated as well as the treatment modalities to be evaluated. As for many other diseases, small animal models using rodents have been widely explored due to their versatility, reproducibility, ease in model standardization, and use of genetically manipulated strains to help uncover disease pathomechanisms. However, the incorporation of clinical standard of care, such as mechanical ventilation, or more subspecialized care, such as extracorporeal membrane oxygenation (ECMO), is extremely challenging in rodent models; therefore, large animal models are most commonly used in these cases (Matute-Bello et al., 2011; Kulkarni et al., 2022).
Porcine models of ARDS have attracted interest because their size and general anatomy is similar to humans, allowing for clinically relevant interventions and measurement techniques (Ballard-Croft et al., 2012; Moodley et al., 2016). Furthermore, the Berlin criteria can be used to evaluate and confirm large animal models due to the fact that a) the timing and type of injury is known, b) the origin of edema can be confirmed by monitoring cardiac parameters and fluid support intake before injury onset, and c) oxygenation levels can be readily measured through serial blood gases. However, chest imaging using CT can be challenging to incorporate due to the short duration of injury models utilized for ARDS, lack of available equipment, or challenges with transport logistics (Hellbach et al., 2018). Therefore, the current gold standard to compensate for the absence of high-resolution chest imaging and to exclude other differential diagnoses is to confirm tissue level injury in animal models by examining the extent of histological tissue damage (Matute-Bello et al., 2008; Wang et al., 2008; Leiphrakpam et al., 2021).
In order to evaluate tissue level injury, histological sections across different time points are required. As artifacts arising from improper preparation can convolute the interpretation of histological findings, careful histological processing of tissue is required to achieve proper insight into the pathological state of the injured tissue (Hsia et al., 2010). The most widely used histopathological processing technique for ALI across species utilizes formalin-fixation and paraffin embedding (FFPE), which provides high morphological and cellular level resolution. In order to generate FFPE samples, the biopsied pieces of tissue need to be properly fixed, followed by tissue processing and paraffin embedding. Next, thin sections are generated and placed on microscopic slides, followed by staining, and finally, microscopy and image analysis. All of these steps are time-consuming and labor intensive, and can thus be prone to variability (Hsia et al., 2010).
As large animal models often generate considerable sample numbers, efficient and reproducible workflows are needed for processing and analyzing histological sections. Furthermore, traditional histological processing uses toxic chemicals for both the environment and the end-user (de Aquino et al., 2016; Kandyala et al., 2010; Purdie et al., 2011). This provided a secondary motivation to the development and implementation of a more sustainable and greener histological workflow, achieving several of the sustainability goals in Agenda 2030 (United Nations, 2015). We focused on goal 3—good health and well-being—and goal 12—responsible consumption and production. Xylene removal from the clearing steps can dramatically reduce the overall amount of xylene used (goal 12) and improve the health and well-being of the histopathologist (goal 3). Therefore, we replaced xylene with isopropanol, which is environmentally and occupationally safer (Falkeholm et al., 2001). When possible, we also used revitalized histological equipment or repurposed commonly available items to make histological processing more accessible to laboratories lacking the resources for some of the high-end and specialized equipment used for histological processing.
Once histological sections are generated, they can be used to validate the extent of injury. As histological analysis can be subjective, the use of semi-quantitative scoring systems is viewed as highly relevant for confirming ARDS in animal models and for evaluating potential therapies (Meyerholz and Beck, 2018). The development of a validated histological score system of ALI in large animal models has been deemed a priority in the field (Kulkarni et al., 2022). Objectively comparing histological injury is known to be challenging, even for highly trained pathologists. Previous histological scoring systems for small animal models relied on the use of scoring multiple randomized fields of view in bright-field microscopy. However, slide scanning technology is becoming more common and accessible to more laboratories. Slide scanning technology therefore opens up new possibilities for histological scoring in a digital format. Therefore, we developed a new histological scoring system for assessing the extent of pulmonary tissue damage in hematoxylin and eosin-stained sections generated from porcine models of ARDS, which could be used by researchers with varying degrees of histological experience. While histological scoring of specific biomarkers can be useful, this becomes challenging in large animal models where molecular tools are not always available. Furthermore, the presence or absence of certain biomarkers may change depending on the source of injury. Therefore, to simultaneously achieve our abovementioned goals, we chose to implement our histological scoring system with hematoxylin and eosin-stained sections generated using green histological processing techniques.
Materials and Reagents
15 mL conical tube (Sarstedt, catalog number: 62.554.502)
Glass petri dish, 12 cm diameter (Thermo Scientific, catalog number: 41042030)
Scalpel blade, no. 21 (Heraco AB, catalog number: 7978)
Scalpel, no. 4, (Heraco AB, catalog number: 7972)
Forceps curved, 2 mm Tip, 12 cm (Agnthos, catalog number: 11003-12)
Paraffin (Histolab, Histowax; catalog number: 00403)
Stainless steel base molds 1.5 × 1.5 × 0.5 cm (VWR International AB, catalog number: 89498-710)
Glass slides (Microscopic slides, Fisher Scientific; catalog number:10149870)
Wheaton Coplin staining jars (Sigma-Aldrich, catalog number: S5766-6EA)
Cover slips 24 × 50 mm (Histolab, Thermo Scientific; catalog number: 06660)
10% neutral-buffered formalin (Sigma-Aldrich, catalog number: HT501128-4L)
Phosphate buffered solution (PBS) (Thermo Scientific, catalog number: 18912014)
Tissue processing/embedding cassettes (Histolab, catalog number: 41701)
Absolute ethanol (VWR International AB; catalog number: BAKR3406.5000)
Isopropanol (Fisher Scientific, catalog number: 15518744)
Xylene (Merck, catalog number: 1330-20-7)
Hematoxylin (Merck, catalog number: 105175)
Glacial acetic acid (Sigma-Aldrich, catalog number: 64-19-7)
Pertex mounting media (Histolab, Thermo Scientific; catalog number: 00871.0500)
Eosin Y solution 0.5% aqueous (Working Solution) (see Recipe 1) (VWR International AB, catalog number: 1098441000)
Graded ethanol solutions and ethanol-isopropanol mixtures (Recipe 2)
Equipment
Tissue processor (Myr, Automated Spin Tissue Processor, model: STP 120) (Figure 1)
Microtome (Leitz, model: 1516 Automated) (Figure 2A)
Slide heater (Wealtec Corp., model: HB-1, catalog number: 1092001) (Figure 2B)
Water bath (JP Selecta, model: N291451, catalog number: 12027874) (Figure 2C)
Cooling tray stored at -20 °C (33 × 27 × 5 cm) (Menuett, model: 009551) (Figure 2D)
Custom-made Paraffin Dispensing Tank (ROYAL Catering, catalog number: 317320191080) retrofitted with a 16 mm diameter and 55 mm external length stainless steel spigot (The Kitchen Lab, Sweden; AM-SIGNSTEK304) (Figure 2E)
Natural convection oven (BINDER, catalog number: ED56 8012-1018)
VS120-S6-096 virtual microscopy slide scanning system (Olympus, Tokyo, Japan)

Figure 1. Spin Tissue Processor (STP 120) showing individual containers numbered 1-12 and the programmable interface.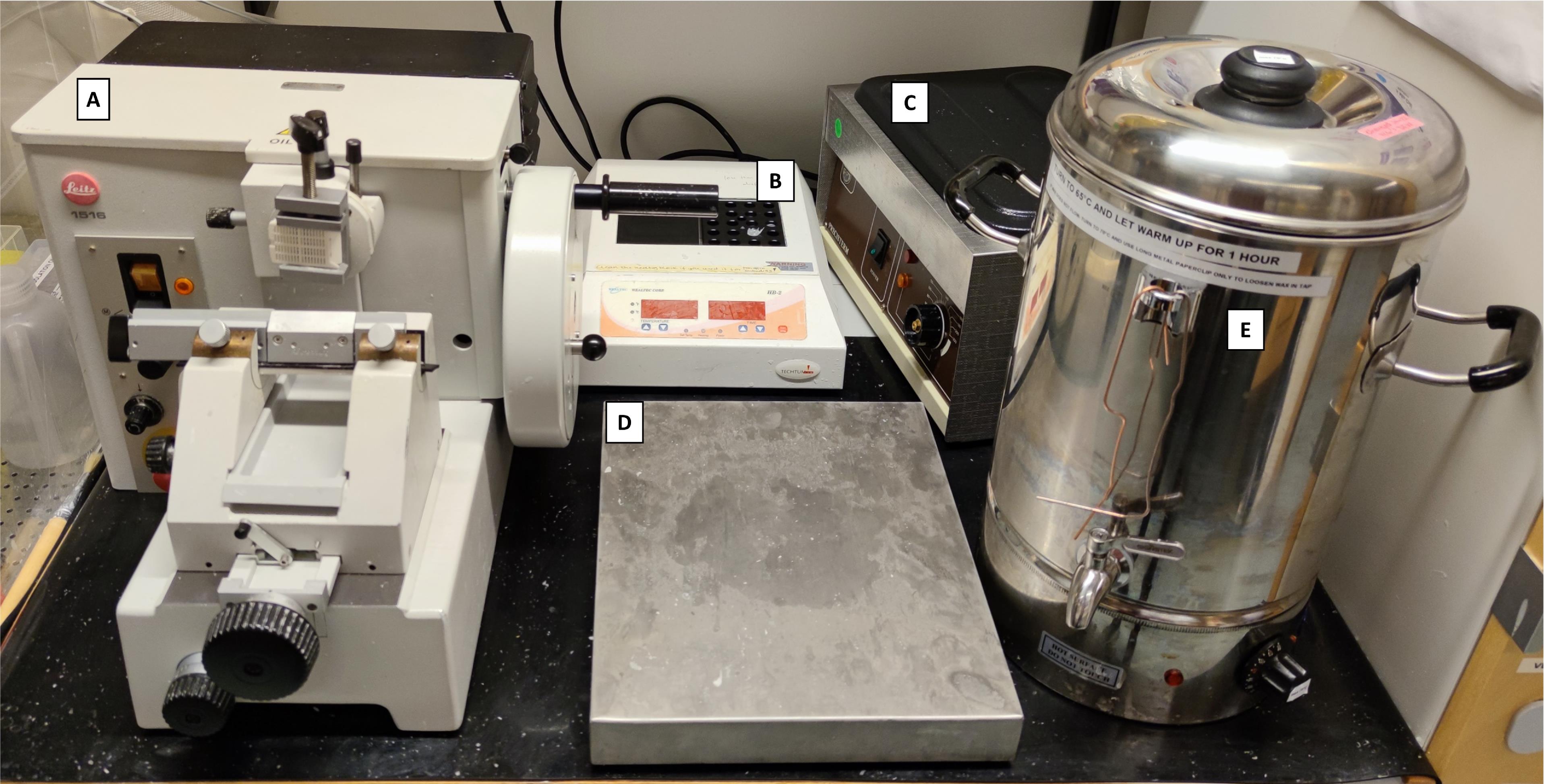
Figure 2. Histopathology area, developed with revitalized, refurbished, and repurposed equipment. A) Manual microtome (revitalized); B) Block heater (refurbished); C) Water bath (revitalized); D) Cooling tray (repurposed); E) Paraffin tank dispenser (water boiler retrofitted with a stainless-steel spigot).
Software
OlyVIA 3.8 (Olympus)
Graphpad Prism 9 (La Jolla, CA)
Optional: Microsoft® PowerPoint® and Microsoft Excel® for Microsoft 365 MSO (Version 2201 Build 16.0.14827.20158) 64-bit
Adobe Acrobat Pro DC version: 21.011.20039.0
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Silva, I. A. N., Gvazava, N., Bölükbas, D. A., Stenlo, M., Dong, J., Hyllen, S., Pierre, L., Lindstedt, S. and Wagner, D. E. (2022). A Semi-quantitative Scoring System for Green Histopathological Evaluation of Large Animal Models of Acute Lung Injury. Bio-protocol 12(16): e4493. DOI: 10.21769/BioProtoc.4493.
分类
细胞生物学 > 组织分析 > 组织形态学
细胞生物学 > 组织分析 > 组织染色
环境生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link