- EN - English
- CN - 中文
Co-differentiation and Co-maturation of Human Cardio-pulmonary Progenitors and Micro-Tissues from Human Induced Pluripotent Stem Cells
人诱导多能干细胞中人心肺祖细胞和微组织的共分化和共成熟
发布: 2022年08月20日第12卷第16期 DOI: 10.21769/BioProtoc.4488 浏览次数: 3287
评审: Gal HaimovichFarah HaqueSrinidhi Rao Sripathy RaoAnonymous reviewer(s)
Abstract
Currently, there are several in vitro protocols that focus on directing human induced pluripotent stem cell (hiPSC) differentiation into either the cardiac or pulmonary lineage. However, these systemsprotocols are unable to recapitulate the critical exchange of signals and cells between the heart and lungs during early development. To address this gap, here we describe a protocol to co-differentiate cardiac and pulmonary progenitors within a single hiPSC culture by temporal specific modulation of Wnt and Nodal signaling. Subsequently, human cardio-pulmonary micro-tissues (μTs) can be generated by culturing the co-induced cardiac and pulmonary progenitors in 3D suspension culture. Anticipated results include expedited alveolarization in the presence of cardiac cells, and segregation of the cardiac and pulmonary μTs in the absence of exogenous Wnt signaling. This protocol can be used to model cardiac and pulmonary co-development, with potential applications in drug testing, and as a platform for expediting the maturation of pulmonary cells for lung tissue engineering.
Keywords: Cardio-pulmonary (心肺)Background
From embryogenesis to adulthood, the heart and lungs are always located in close proximity to each other. This suggests the presence of inter-organ communication that influences their respective developmental programs (Hoffmann et al., 2009; Arora et al., 2012; Peng et al., 2013; Steimle et al., 2018). This idea is well supported by prior work in animal models. For instance, Wnt ligands from the heart field are crucial for foregut endoderm specification (Steimle et al., 2018) and, subsequently, the Hedgehog ligand secreted by the specified lung endoderm is critical for atrial septation (Hoffmann et al., 2009). Additionally, cardio-pulmonary progenitors originating from the second heart field are required for the formation of pulmonary vasculature (Peng et al., 2013). While such work in animal models indicate extensive crosstalk between the developing heart and lungs, the relevance of these findings to human development remains unknown.
Due to limited access to human embryos and ethical concerns, there is a critical lack of platforms that allow for the translation of animal studies into an understanding of the interrelationship between the heart and lungs during human development. Advancements in induced pluripotent stem cell (iPSC) technology have demonstrated the feasibility for direct differentiation of either cardiac (Kattman et al., 2011; Lian et al., 2012, 2015; Mummery et al., 2012; Burridge et al., 2014; Lee et al., 2017) or pulmonary (Wong et al., 2012; Gotoh et al., 2014; Huang et al., 2014; Dye et al., 2015; Chen et al., 2017; Jacob et al., 2017) lineages from human iPSCs (hiPSCs). However, it remains challenging to investigate the mutual interaction between the mesoderm-derived heart and endoderm-derived lung lineages during their specification from hiPSCs. While prior studies have shown that pluripotent stem cells can be differentiated into mesendoderm (Martyn et al., 2019)—the precursor for mesoderm and endoderm, which ultimately can be directed into the cardiac and pulmonary lineages, respectively—we explored and validated the possibility of differentiating hiPSCs into both heart and lung cells within the same culture by modulating the paracrine signaling required for inducing both lineages.
This protocol describes a methodology for simultaneously generating cardiac and pulmonary progenitors from hiPSCs, involving the induction of the primitive streak, definitive endoderm/mesoderm, anterior foregut endoderm/cardiac mesoderm, and finally cardiac and pulmonary progenitors within 15 days of differentiation. In this protocol we used an hiPSC line carrying both NKX2.1-GFP and SFTPC-TdTomato fluorescent reporters to track the emergence of early pulmonary progenitors and alveolar type 2 cells. 3D suspension culture of the co-induced cardiac and pulmonary progenitors led to expedited alveolarization within 3 days. Further, the withdrawal of Wnt agonist (CHIR99021) facilitated cardio-pulmonary tissue segregation, generating pulmonary-only and cardiac-only μTs (Ng et al., 2022). This protocol can be used to generate human cardio-pulmonary progenitors for investigating their co-development and crosstalk in vitro, as well as for drug testing.
Materials and Reagents
For Cell Culture
96-well plate (Corning, catalog number: 3698)
6-well plate (Corning, catalog number: 3516)
Ultra-low adherence 24-well plate (Greiner Bio-One, catalog number: 662970)
Ultra-low adherence 96-well plate (Greiner Bio-One, catalog number: 650979)
hiPSCs BU3-NGST [a gift from Dr. Darrell Kotton’s Laboratory, Boston University; users can request this cell line from Dr. Kotton’s Lab (Jacob et al., 2017)]
mTeSR-Plus (Stem Cell Technologies, catalog number: 05825)
hESC-qualified Matrigel Basement Membrane Matrix (Corning, catalog number: 354234)
ReLeSRTM (Stem Cell Technologies, catalog number: 05873)
StemPro® Accutase® Cell Dissociation Reagent (Thermo Fisher Scientific, catalog number: A1110501)
Dulbecco’s Phosphate-Buffered Saline (DPBS) (Corning, catalog number: 45000-430)
RPMI-1640 (Corning, catalog number: 10-040-CV)
DMEM/F12 (Corning, catalog number: 10-090-CV)
GlutaMAXTM (Thermo Fisher Scientific, catalog number: 35050061)
B27 Supplement (minus insulin) (Thermo Fisher Scientific, catalog number: A1895601)
B27 Supplement (Complete) (Thermo Fisher Scientific, catalog number: 12587-010)
TrypLE Express (Thermo Fisher Scientific, catalog number: 12605028)
HycloneTM Fetal Bovine Serum (FBS) (Cytiva, catalog number: SH30071.03)
Penicillin-Streptomycin (Pen/Strep), 10,000 U/mL (Thermo Fisher Scientific, catalog number: 15140148)
Trypan Blue (VWR, catalog number: K940-100ML)
Media Recipes/Composition (see Recipes)
For immunocytochemistry
Methanol (Fisher Chemical, catalog number: BPA412-1)
Bovine Serum Albumin (BSA) (Fisher BioReagents, catalog number: BP9706-100)
Phosphate Buffer Saline (PBS) 20× (Growcells, catalog number: MRGF-695-010L)
Triton X-100 (Sigma-Aldrich, catalog number: X100-500ML)
Antibodies
Rabbit monoclonal anti-NKX2.1 (Abcam, catalog number: ab76013)
Goat polyclonal anti-NKX2.5 (R&D Systems, catalog number: AF2444)
Donkey anti-rabbit IgG (H+L) Alexa Fluor 488 (Thermo Fisher Scientific, catalog number: A21206)
Donkey anti-goat IgG (H+L) Alexa Fluor 647 (Thermo Fisher Scientific, catalog number A21447)
Growth factors and small molecules
Recombinant human keratinocyte growth factor (KGF) (PeproTech, catalog number: 100-19)
Y27632 (Cayman Chemical, catalog number: 1000558310)
CHIR99021 (Reprocell, catalog number: 04000402)
A83-01 (Sigma-Aldrich, catalog number: SML0788)
IWP4 (Tocris, catalog number: 5214)
All-trans retinoic acid (Cayman, catalog number: 11017)
Dexamethasone (Sigma-Aldrich, catalog number: D4902)
8-bromoadenosine 3’,5’-cyclic monophosphate sodium salt (cAMP) (Sigma-Aldrich, catalog number: B7880)
3-Isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, catalog number I5879)
Equipment
EVOS FL Auto 2 Imaging System (Thermo Fisher Scientific, catalog number: AMAFD2000)
Thermo ScientificTM SorvallTM LegendTM X1 Centrifuge (Thermo Fisher Scientific, catalog number: 75-218-382)
Thermo ScientificTM 1300 Series Class II, Type A2 Biological Safety Cabinet (Thermo Fisher Scientific, catalog number: 13-261-306)
CO2 Resistant Shaker (Thermo Fisher Scientific, catalog number: 88881103)
FisherbrandTM IsotempTM CO2 Incubator (Thermo Fisher Scientific, catalog number: 11-676-604)
25 cu. ft. (708 L) Reach-in IR CO2 Incubator (Caron Products, catalog number: 7400-25-1)
Software
ImageJ Version 1.8.0.182 (National Institutes of Health, https://imagej.nih.gov/ij/download.html)
Procedure
Matrigel Coating
Note: All steps are performed under class II biological safety cabinet, unless otherwise stated.
Thaw a -80 °C aliquot of Matrigel (100 µL) by placing it in a 4 °C fridge for 1 h.
Prepare the 1% (v/v) working solution by diluting the thawed Matrigel (100 µL) in 10 mL of DMEM/F12.
Add the Matrigel working solution into each well of the desired multi-well plate: 100 µL for the 96-well plate format, and 1.5 mL for the 6-well plate format.
Incubate the plate at 37 °C for at least 3 h.
Prior to use, aspirate the diluted Matrigel solution and use without washing the well.
BU3-NGST cell maintenance and passaging
Note: All steps are performed under class II biological safety cabinet, unless otherwise stated.
Prior to cell passaging, coat the wells of a 6-well plate with 1% (v/v) Matrigel for at least 3 h at 37 °C.
Remove mTeSR-Plus from Day 5 sub-confluent BU3-NGST cultured on a 6-well tissue culture plate and wash twice with 4 mL of DPBS.
Add 2 mL of ReLeSR and incubate for 15 s. Remove the ReLeSR and incubate the cells at 37 °C for an additional 5 min.
Collect the cells by adding 2 mL of fresh mTeSR-Plus and centrifuge at 300× g for 3 min.
Discard the supernatant and resuspend the cells in 1 mL of fresh mTeSR-Plus.
Passage the cells at ratios of 1:10 to 1:20.
Replenish 2 mL of mTeSR-Plus medium on Day 2, and 4 mL of medium on Day 4.
Normally, cells will be ready to passage again on Day 5.
Plating of BU3-NGST for differentiation
Note: All steps are performed under class II biological safety cabinet, unless otherwise stated. The following steps describe the procedure for obtaining dissociated cells from one well of a 6-well plate.
Prior to cell plating, coat the wells of a 96-well plate with 1% (v/v) Matrigel for at least 3 h at 37 °C.
Remove mTeSR-Plus from Day-5 sub-confluent BU3-NGST (Figure 1A) cultured on a 6-well tissue culture plate and wash twice with 4 mL of DPBS.
To dissociate the hiPSCs into single cells, add 3 mL of pre-warmed Accutase (avoid repeating the freeze-thaw cycle), and incubate for 5 min at 37 °C. Transfer dissociated BU3-NGST into 3 mL of fresh mTeSR-Plus and centrifuge at 300 × g for 3 min.
Discard supernatant and resuspend the cell pellet with 1 mL fresh mTeSR-Plus supplemented with 10 μM Y27632. Gently pipette up and down 10 times to break the cell pellet into single-cell suspension.
Perform cell counting using a hemacytometer by diluting the cells in Trypan Blue at ratio 1:2.
Plate 150,000 cells/cm2 per well in 100 μL of mTeSR-Plus supplemented with 10 μM Y27632 (Figure 1B).
Note: The cells will be ready for differentiation the following day.
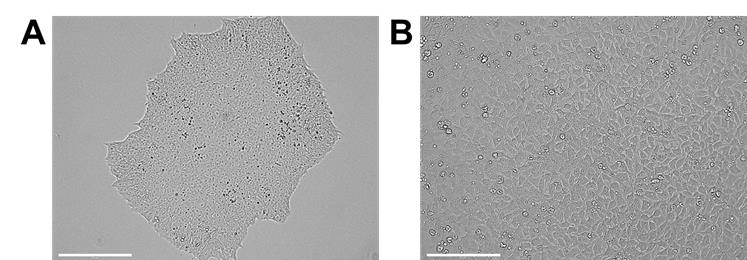
Figure 1. Undifferentiated iPSCs in culture. A. Undifferentiated hiPSC colony. B. hiPSCs after one day of single-cell plating (scale bars = 200 μm).Differentiation from hiPSCs to human cardio-pulmonary progenitors
Note: All steps are performed under class II biological safety cabinet unless otherwise stated. The following steps describe the procedure for differentiating hiPSCs from one well of a 96-well plate.
On the day of induction (Day 0, one day after single-cell plating), remove cell culture medium and add 200 μL of 7 μM CHIR99021 in mTeSR-Plus supplemented with 10 μM Y27632. Refresh the medium on Day 1.
Note: The CHIR99021 concentration required for effective and balanced cardio-pulmonary induction can be cell line dependent. Perform optimization of CHIR99021 concentration as needed.
On Day 2, remove the medium and wash the culture twice with 200 μL pre-warmed RPMI-1640. Add 100 μL pre-warmed medium (composed of RPMI-1640, 1× GlutaMAX, 1× B27 minus insulin, 10 μM Y27632, and 1% Pen/Strep). Refresh the medium on Day 3.
Note: Check for cell viability at this stage. Massive cell death will lead to poor co-differentiation outcome.
On Days 4–7, remove the medium and add 100 μL of pre-warmed medium supplemented with 1 μM A83-01 and 5 μM IWP4. Refresh the medium daily.
On Day 8, remove the medium and wash the culture once with 100 μL pre-warmed RPMI-1640. During Days 8–14, add 100 μL of pre-warmed medium (composed of RPMI-1640, 1× GlutaMAX, 1× B27 complete, and 1% Pen/Strep) supplemented with 3 μM CHIR99021 and 100 nM retinoic acid, and refresh the medium daily.
Note: The appearance of NKX2.1-GFP, which indicates early lung progenitors, can be observed from Day 10 onwards (Figure 2A).
To assess the co-differentiation efficiency, fix the differentiated human cardio-pulmonary progenitors on Day 15, and check for cardiac (NKX2.5) and pulmonary (NKX2.1) markers using immunocytochemistry (Figure 2C–2D) (see section G). Quantify NKX2.1-GFP lung progenitor efficiency on Day 15 by flow cytometry (Figure 2B) (see section H).
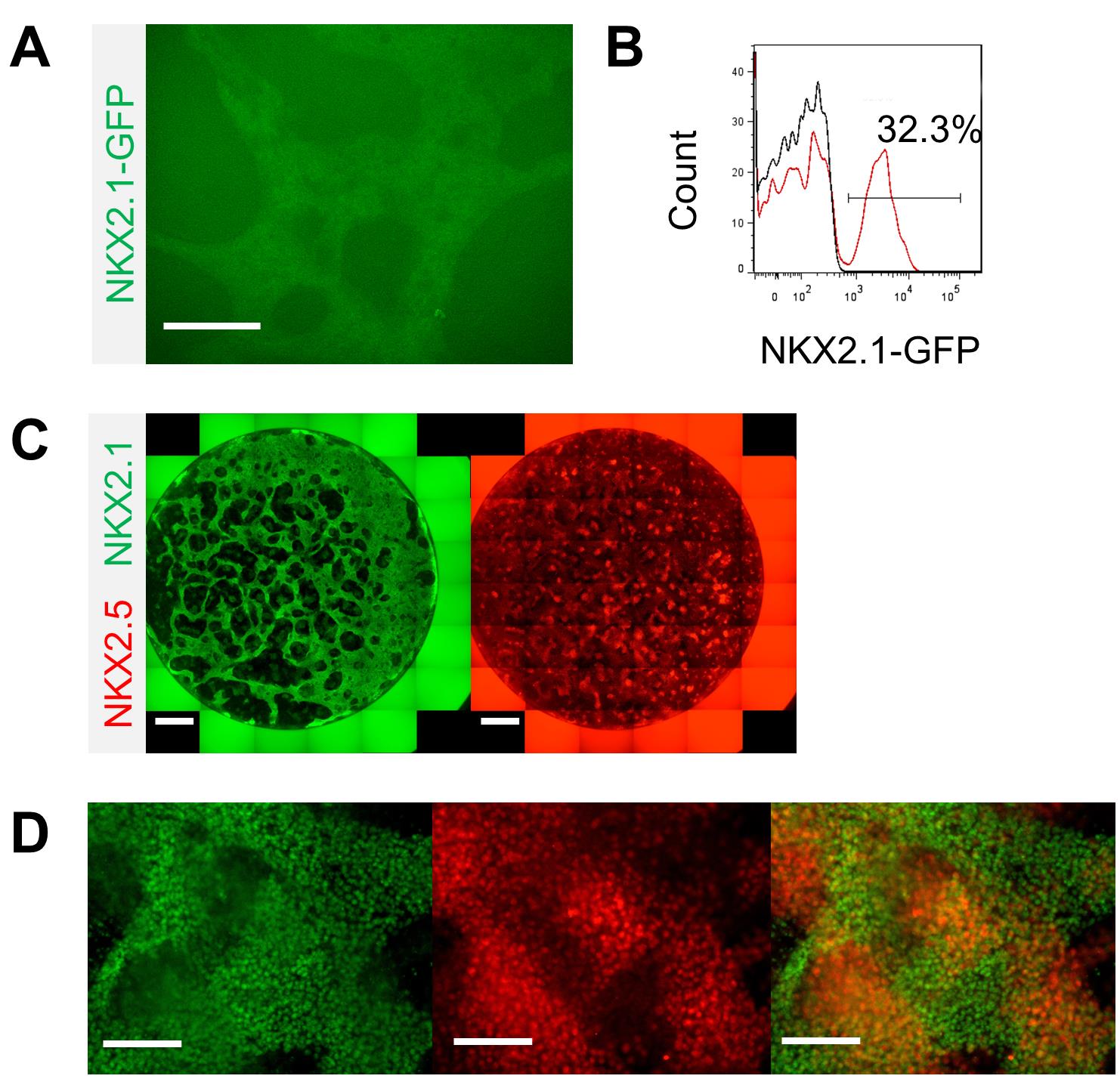
Figure 2. Characterization of the co-induced human cardio-pulmonary progenitors. A. GFP expression indicating NKX2.1-expressing pulmonary progenitors (scale bar = 125 μm). B. Quantification of NKX2.1-GFP lung progenitors on Day 15 by flow cytometry. C, D. Immunofluorescence staining of NKX2.1 (pulmonary) and NKX2.5 (cardiac) (scale bars for panel C = 250 μm; scale bars for panel D = 125 μm).Generation of human cardio-pulmonary microtissues (μTs)
On Day 15, trypsinize at least 3 wells (96-well plate format) of the human cardio-pulmonary progenitors using 100 μL TrypLE Express and incubate at 37 °C for 15 min.
Neutralize the TrypLE Express by adding 200 μL RPMI-1640 supplemented with 10% FBS.
Centrifuge at 300 × g for 3 min.
Resuspend the cell pellet in alveolar maturation medium (comprising RPMI-1640, 1× GlutaMAX, 1% Pen/Strep, 1× B27 complete, 3 μM CHIR99021, 10 ng/mL KGF, 50 nM dexamethasone, 0.1 mM cAMP, 0.1 mM IBMX, and 10 μM Y27632).
Plate 500k cells into one well of an ultra-low adhesion 24-well plate in 500 μL of alveolar maturation medium and incubate on an orbital shaker at 125 rpm to generate μTs.
On Day 16, perform full medium change with alveolar maturation medium without 10 μM Y27632. Tilt the plate at a small angle to collect all μTs at one corner, then carefully aspirate and remove the majority of medium without losing the μTs. Refresh the medium on Day 17.
On Day 18, check for SFTPC-TdTomato using a fluorescence microscope (Figure 3).
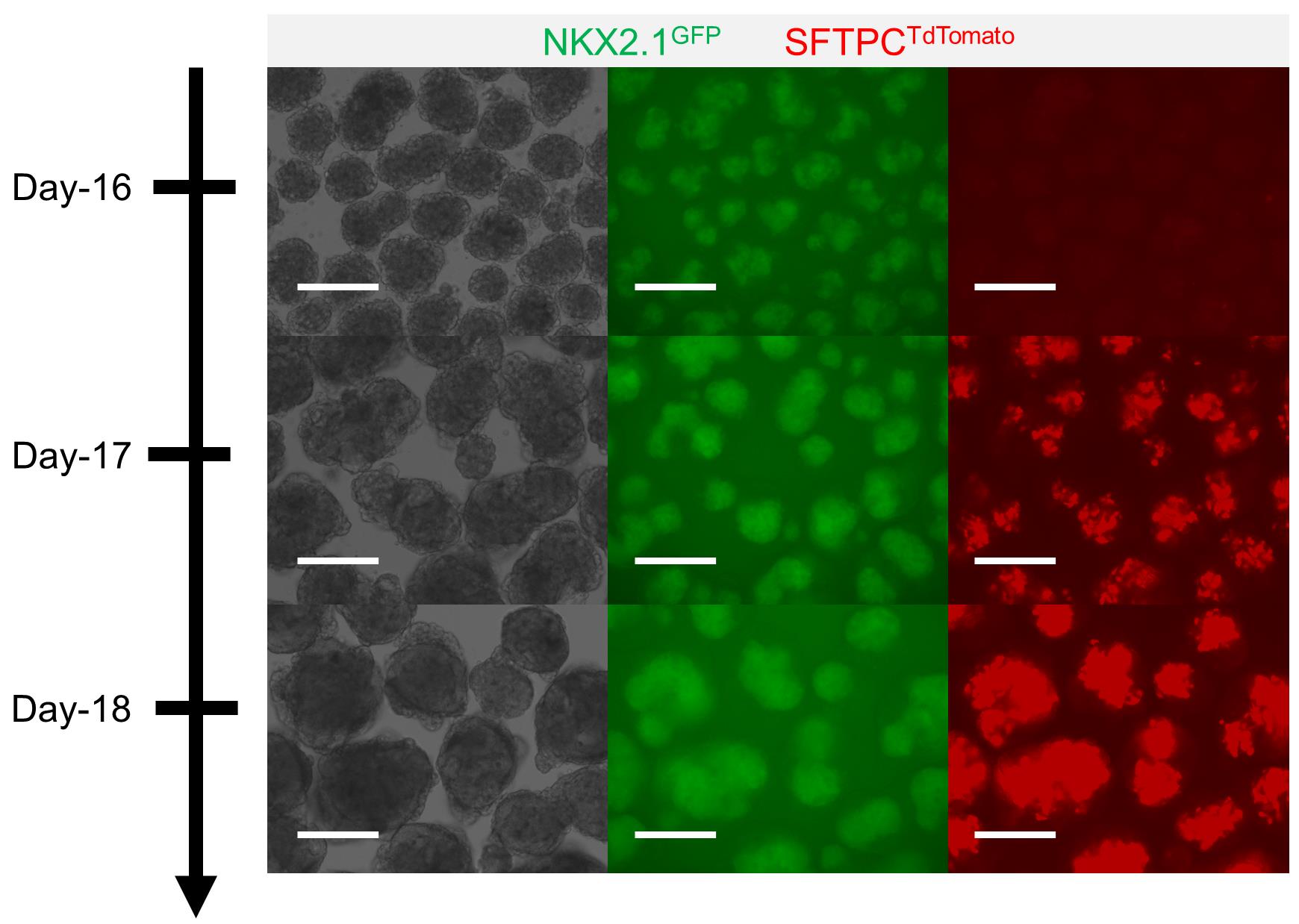
Figure 3. Formation of human cardio-pulmonary μTs. Alveolar type 2 cell maturation identified through the emergence of SFTPC-TdTomato fluorescence signal on Day 17; the signal becomes stronger on Day 18 (scale bars = 125 μm).Segregation of human cardio-pulmonary μTs
On Day 18, transfer one μT from the ultra-low adhesion 24-well plate into each well of an ultra-low adhesion 96-well plate.
Note: The μTs are visible to the naked eye. By inserting a 100 μL pipette tip close enough to the μTs, one can transfer a single μT from the well. Examine under the microscope to confirm.
Replace the medium with alveolar maturation medium without CHIR99021 or Y27632.
On Days 19–23, add 20 μL of cell culture water into each well to compensate medium evaporation and incubate for at least 10 min to allow complete mixture.
Note: Evaporation of the culture medium is inevitable. This step is crucial to prevent salt from accumulating over time in the culture medium following evaporation.
Remove 50 μL of medium and replenish with 50 μL of fresh medium.
On Day 23, look for segregated pulmonary μTs expressing GFP and contracting cardiac μTs (Figure 4).
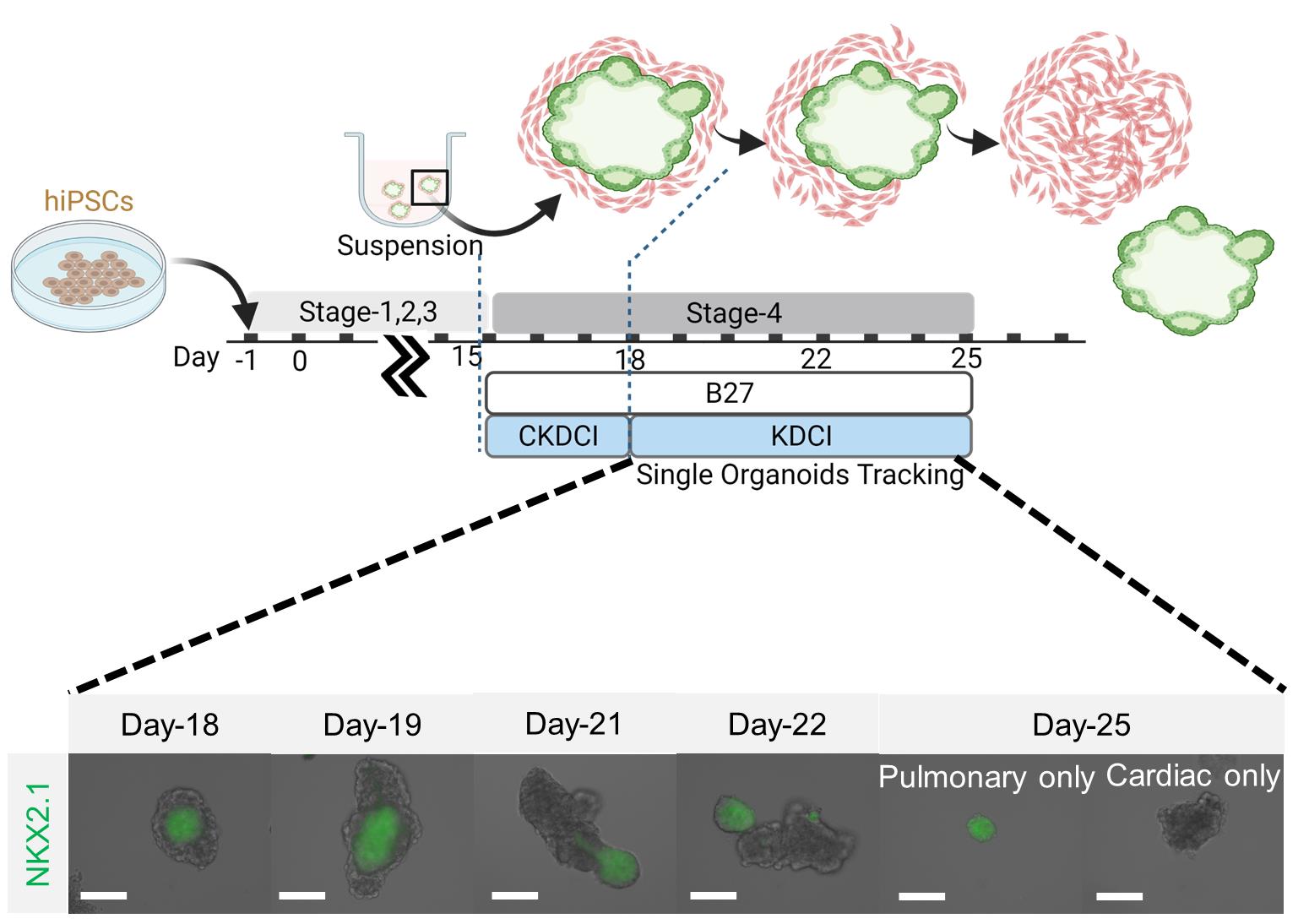
Figure 4. Segregation of human cardio-pulmonary μTs. μTs before and after segregation (scale bars = 125 μm). Schematic diagram adapted from Ng et al. (2022).Immunocytochemistry
Note: The following steps describe the staining protocol for cells plated on one well of a 96-well plate.
Fix cells with 100 μL of ice-cold methanol for 20 min.
Discard methanol and air-dry the samples for at least 1 h.
Note: Complete air-dried samples can be rehydrated in PBS and kept at 4 °C for up to a week prior to immunocytochemistry staining. Insufficient air-drying may compromise staining outcome.
Permeabilize cells with 1% (v/v) Triton X-100 in PBS at room temperature for 20 min.
Discard the permeabilization buffer and wash three times with PBS.
Block the cells with 1% BSA diluted in PBS for 30 min at room temperature.
Prepare working stocks of primary antibodies by dilution in 1% BSA (in PBS). See Table 1 for recommended primary antibodies and their working dilutions for each cell type.
Incubate the cells in 30 μL of primary antibodies overnight at 4 °C.
Wash the cells three times with PBS, add 30 μL of secondary antibodies diluted in 1% BSA (in PBS), and incubate at room temperature for 1 h. See Table 1 for recommended secondary antibodies and dilutions.
Wash three times with PBS and leave 300 μL of PBS in the well following the final wash. The cells are then ready for imaging.
Table 1. Antibody dilution
Antibody Dilution Anti-NKX2.1 (rabbit monoclonal) 1:500 Anti-NKX2.5 (goat polyclonal) 1:500 Donkey anti-rabbit IgG (H+L), Alexa Fluor 488 1:500 Donkey anti-goat IgG (H+L), Alexa Fluor 647 1:500
Flow cytometry
Dissociate Day 15 co-differentiated cells in TrypLE Express for 15 min at 37 °C.
Neutralize TrypLE Express using RPMI-1640 supplemented with 10% FBS and centrifuge at 300 × g for 3 min.
Discard supernatant and resuspend cells in incubation medium (composed of DPBS and 1% FBS).
Analyze the percentage of cells expressing GFP using BD FACSAria II.
Data analysis
Detailed analysis of hiPSC-derived cardio-pulmonary progenitors and their maturation can be found in the open access article recently published by our group (Ng et al., 2022).
Notes
The hiPSC line and initial cell seeding density may affect the outcome of the differentiation efficiency and the sensitivity of cells to CHIR99021 treatment. For different hiPSC lines, it is recommended to explore different cell densities and CHIR99021 concentrations to identify the optimal combination for cardio-pulmonary co-induction. When the differentiation efficiency is compromised, check the quality of the hiPSC maintenance culture. It is important to spot any spontaneous differentiation, over confluency, suspicious cell death, or potential microorganism contamination. It is recommended to thaw a new vial of hiPSCs every 6-12 months to ensure reproducible cellular performance. It is also recommended to wash the cells with basal medium (such as RPMI-1640) at least once prior to switching to a different medium for the next stage of differentiation.
Recipes
Media Recipes/Composition
Note: Differentiation medium can be stored at 4 °C for up to 1 week. Aliquot the needed amount of medium and pre-warm in a 37 °C water bath for 5–10 min before using (beware of any undissolved stock solution).
Media Base Small molecules/Growth Factors Final Concentration Stage 1: Day 0–1 mTeSR Plus CHIR99021 7 μM Y27632 10 μM Stage 1: Days 2–3 RPMI-1640 Y27632 10 μM B-27 minus insulin (1×) GlutaMAX (1×) Stage 2: Days 4–7 RPMI-1640 A83-01 1 μM B-27 complete (1×) IWP4 5 μM GlutaMAX (1×) Y27632 10 μM Stage 3: Days 8–14 RPMI-1640 CHIR99021 3 μM B27 complete (1×) Retinoic acid 100 nM GlutaMAX (1×) Stage 4: Days 15–17 RPMI-1640 CHIR99021 3 μM B27 complete (1×) KGF 10 ng/mL GlutaMAX (1×) Dexamethasone 50 nM cAMP 0.1 mM IBMX 0.1 mM Stage 4: Day 18 onwards RPMI-1640 KGF 10 ng/mL B27 complete (1×) Dexamethasone 50 nM GlutaMAX (1×) cAMP 0.1 mM IBMX 0.1 mM
Acknowledgments
This work was supported by Samuel & Emma Winters Foundation A025662 (to X.R.), National Science Foundation CBET2145181 (to X.R.), and the Department of Biomedical Engineering and College of Engineering at Carnegie Mellon University. This protocol was adapted from our previous work (Ng et al., 2022).
Competing interests
W.H.N, and X.R. have a provisional patent application (No. 63/124422; “Methods for simultaneous cardio-pulmonary differentiation and alveolar maturation from human pluripotent stem cells”) related to this study.
References
- Arora, R., Metzger, R. J. and Papaioannou, V. E. (2012). Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet 8(8): e1002866.
- Burridge, P. W., Matsa, E., Shukla, P., Lin, Z. C., Churko, J. M., Ebert, A. D., Lan, F., Diecke, S., Huber, B., Mordwinkin, N. M., et al. (2014). Chemically defined generation of human cardiomyocytes. Nat Methods 11(8): 855-860.
- Chen, Y. W., Huang, S. X., de Carvalho, A., Ho, S. H., Islam, M. N., Volpi, S., Notarangelo, L. D., Ciancanelli, M., Casanova, J. L., Bhattacharya, J., et al. (2017). A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19(5): 542-549.
- Dye, B. R., Hill, D. R., Ferguson, M. A., Tsai, Y. H., Nagy, M. S., Dyal, R., Wells, J. M., Mayhew, C. N., Nattiv, R., Klein, O. D., et al. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4: e05098.
- Gotoh, S., Ito, I., Nagasaki, T., Yamamoto, Y., Konishi, S., Korogi, Y., Matsumoto, H., Muro, S., Hirai, T., Funato, M., et al. (2014). Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports 3(3): 394-403.
- Hoffmann, A. D., Peterson, M. A., Friedland-Little, J. M., Anderson, S. A. and Moskowitz, I. P. (2009). sonic hedgehog is required in pulmonary endoderm for atrial septation. Development 136(10): 1761-1770.
- Huang, S. X., Islam, M. N., O'Neill, J., Hu, Z., Yang, Y. G., Chen, Y. W., Mumau, M., Green, M. D., Vunjak-Novakovic, G., Bhattacharya, J., et al. (2014). Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32(1): 84-91.
- Jacob, A., Morley, M., Hawkins, F., McCauley, K. B., Jean, J. C., Heins, H., Na, C. L., Weaver, T. E., Vedaie, M., Hurley, K., et al. (2017). Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 21(4): 472-488 e410.
- Kattman, S. J., Witty, A. D., Gagliardi, M., Dubois, N. C., Niapour, M., Hotta, A., Ellis, J. and Keller, G. (2011). Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8(2): 228-240.
- Lee, J. H., Protze, S. I., Laksman, Z., Backx, P. H. and Keller, G. M. (2017). Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations. Cell Stem Cell 21(2): 179-194 e174.
- Lian, X., Bao, X., Zilberter, M., Westman, M., Fisahn, A., Hsiao, C., Hazeltine, L. B., Dunn, K. K., Kamp, T. J. and Palecek, S. P. (2015). Chemically defined, albumin-free human cardiomyocyte generation. Nat Methods 12(7): 595-596.
- Lian, X., Hsiao, C., Wilson, G., Zhu, K., Hazeltine, L. B., Azarin, S. M., Raval, K. K., Zhang, J., Kamp, T. J. and Palecek, S. P. (2012). Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A 109(27): E1848-1857.
- Martyn, I., Brivanlou, A. H. and Siggia, E. D. (2019). A wave of WNT signaling balanced by secreted inhibitors controls primitive streak formation in micropattern colonies of human embryonic stem cells. Development 146(6): dev172791.
- Mummery, C. L., Zhang, J., Ng, E. S., Elliott, D. A., Elefanty, A. G. and Kamp, T. J. (2012). Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 111(3): 344-358.
- Ng, W. H., Johnston, E. K., Tan, J. J., Bliley, J. M., Feinberg, A. W., Stolz, D. B., Sun, M., Wijesekara, P., Hawkins, F., Kotton, D. N., et al. (2022). Recapitulating human cardio-pulmonary co-development using simultaneous multilineage differentiation of pluripotent stem cells. Elife 11: e67872.
- Peng, T., Tian, Y., Boogerd, C. J., Lu, M. M., Kadzik, R. S., Stewart, K. M., Evans, S. M. and Morrisey, E. E. (2013). Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature 500(7464): 589-592.
- Steimle, J. D., Rankin, S. A., Slagle, C. E., Bekeny, J., Rydeen, A. B., Chan, S. S., Kweon, J., Yang, X. H., Ikegami, K., Nadadur, R. D., et al. (2018). Evolutionarily conserved Tbx5-Wnt2/2b pathway orchestrates cardiopulmonary development. Proc Natl Acad Sci U S A 115(45): E10615-E10624.
- Wong, A. P., Bear, C. E., Chin, S., Pasceri, P., Thompson, T. O., Huan, L. J., Ratjen, F., Ellis, J. and Rossant, J. (2012). Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30(9): 876-882.
文章信息
版权信息
Ng et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ng, W. H., Varghese, B. and Ren, X. (2022). Co-differentiation and Co-maturation of Human Cardio-pulmonary Progenitors and Micro-Tissues from Human Induced Pluripotent Stem Cells. Bio-protocol 12(16): e4488. DOI: 10.21769/BioProtoc.4488.
- Ng, W. H., Johnston, E. K., Tan, J. J., Bliley, J. M., Feinberg, A. W., Stolz, D. B., Sun, M., Wijesekara, P., Hawkins, F., Kotton, D. N., et al. (2022). Recapitulating human cardio-pulmonary co-development using simultaneous multilineage differentiation of pluripotent stem cells. Elife 11: e67872.
分类
生物工程 > 生物医学工程
发育生物学 > 形态建成 > 器官形成
细胞生物学 > 细胞工程 > 组织工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link