- EN - English
- CN - 中文
Fluorescence Imaging of 3D Cell Models with Subcellular Resolution
具有亚细胞分辨率的 3D 细胞模型的荧光成像
发布: 2022年07月20日第12卷第14期 DOI: 10.21769/BioProtoc.4469 浏览次数: 5086
评审: Gal HaimovichPradeep Kumar BhaskarSarajo Mohanta

相关实验方案
采用 Davidson 固定液和黑色素漂白法优化小鼠眼组织切片的免疫组化染色
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
2025年11月20日 1562 阅读
Abstract
Over the past years, research has made impressive breakthroughs towards the development and implementation of 3D cell models for a wide range of applications, such as drug development and testing, organogenesis, cancer biology, and personalized medicine. Opposed to 2D cell monolayer culture systems, advanced 3D cell models better represent the in vivo physiology. However, for these models to deliver scientific insights, appropriate investigation techniques are required. Despite the potential of fluorescence microscopy to visualize these models with high spatial resolution, sample preparation and imaging assays are not straightforward. Here, we provide different protocols of sample preparation for fluorescence imaging, for both matrix-embedded and matrix-free models (e.g., organoids and spheroids, respectively). Additionally, we provide detailed guidelines for imaging 3D cell models via confocal multi-photon fluorescence microscopy. We show that using these protocols, images of 3D cell culture systems can be obtained with sub-cellular resolution.
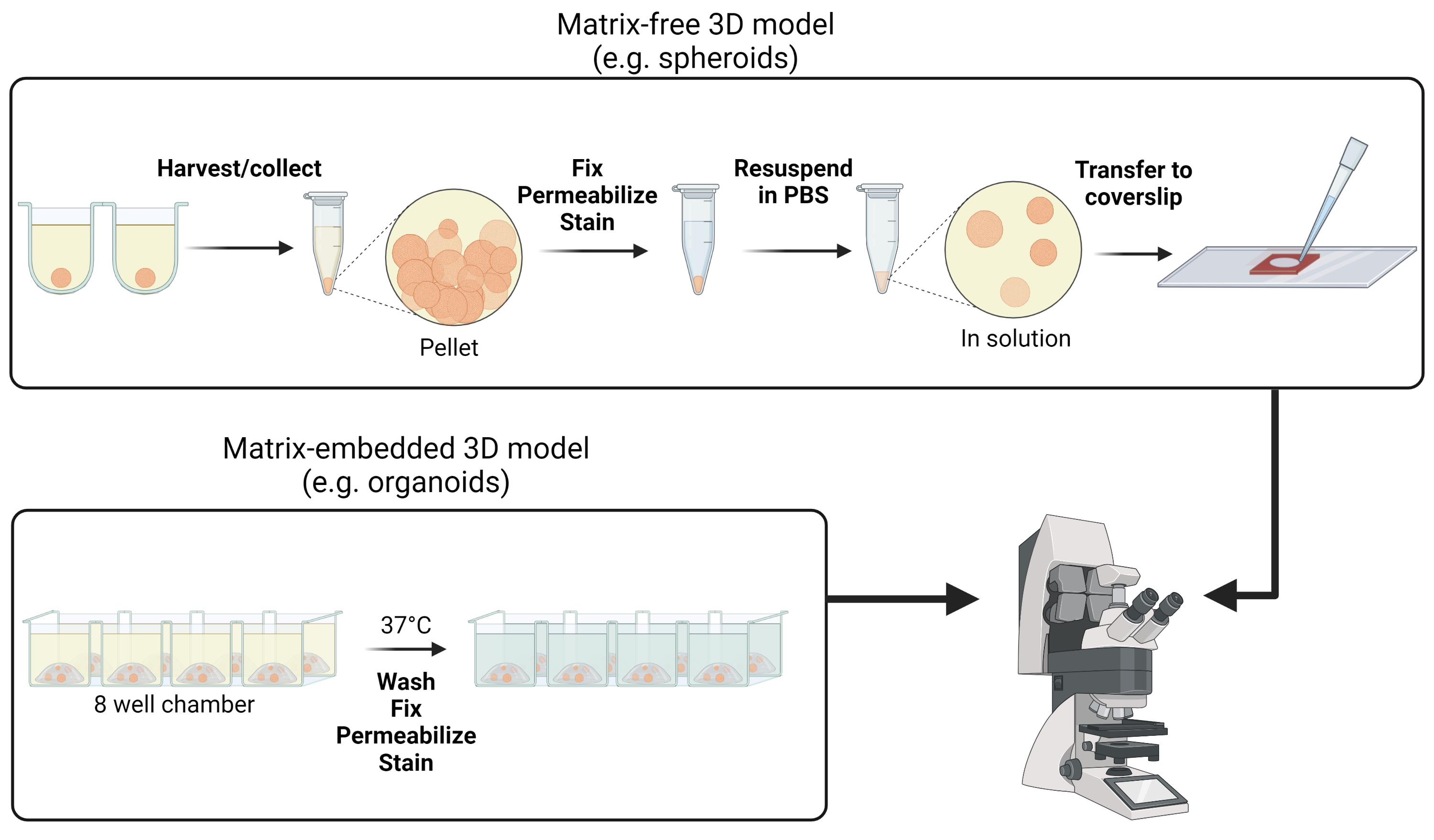
Graphical abstract:
Background
In recent years, 3D cell models have gained increasing attention in a wide variety of research areas. By mimicking physiological conditions, 3D cell models enabled obtaining more detailed information on several biological processes, from general human biology to disease progression and drug delivery. One of the most popular 3D cell models is spheroids, which are usually formed from small cell aggregates that proliferate further into dense cell clusters (Lazzari et al., 2017; Van Zundert et al., 2020). The spheroids primarily used in cancer research are obtained from cancer cells lines and are often referred to as multicellular tumor spheroids. These models have been proven to be more physiologically relevant than 2D monolayer culture systems, as they present key features of solid tumor tissue (Pinto et al., 2020).
The formation of spheroids is relatively straightforward as they require standard growth medium, and several well-established methods are available (Souza et al., 2010; Foty, 2011; Velasco et al., 2020). The growth of organoids, on the other hand, requires special growth factors and the support of a hydrogel scaffold. Organoids are grown from stem cells that are encapsulated in a supporting matrix, usually Matrigel®, and represent a more complex 3D model. When provided with a specific conditioned medium, stem cells encapsulated in biomimetic hydrogels self-organize into miniature organ-like structures (Boretto et al., 2017; Schutgens and Clevers, 2020).
Both organoids and spheroids are relatively large three-dimensional structures (ranging from 200 to 1,000 μm in diameter), which hampers direct imaging of (sub-)cellular organization, especially in optical microscopy, where the mismatch of the refractive indices between the different cellular compartments, causes a high level of scattering in dense multicellular structures. Together with the light absorption by living matter, this implies a limited penetration of the excitation light and reduced signal from the inside the 3D cell models (Edwards et al., 2020). Although optical clearing methods (DISCO, CUBIC, CLARITY, etc.) can significantly enhance the fluorescence signal obtained from thick samples by improving light penetration, they are usually performed under harsh conditions, which often result in loss of cell morphology or sample damage (Ertürk et al., 2012; Tomer et al., 2014; Dekkers et al., 2019). Alternatively, thick samples can be physically sectioned, and the images acquired from each consecutive section can be used to reconstruct the 3D model. However, this method is time-consuming, and artifacts are easily introduced upon sample cutting (Erickson et al., 2011; Bolduan et al., 2020).
Here, we propose a method for performing fluorescence microscopy on 3D cell culture samples without invasive sample processing. Although samples are prepared without optical clearing or sectioning, fluorescence images with sub-cellular resolution can still be achieved by using multi-photon microscopy. We illustrate both an immunofluorescence staining and a staining with small organic molecules, and provide optimized staining protocols for both scaffold-free and scaffold embedded 3D models. The 3D cell culture systems maintain their native morphology and cellular composition, thereby avoiding biased observations. While in these protocols the samples are imaged using two-photon microscopy, the same sample preparation can also be used for single-photon excitation (albeit with reduced light penetration). After performing the 3D reconstruction of the collected images, a detailed three-dimensional view of the whole spheroid or organoid model can be achieved.
Materials and Reagents
Common for both protocols
1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 0030125150)
Aluminum foil
Dulbecco’s phosphate-buffered saline (DPBS) 1× (ThermoFisher Scientific, GibcoTM, catalog number: 14190144)
Formaldehyde (w/v) 16%, methanol-free (ThermoFisher Scientific, Pierce, catalog number: 28908)
Triton X-100 (Sigma-Aldrich, catalog number: T8787)
Bovine serum albumin (BSA; Sigma-Aldrich, catalog number: A2058)
Fixation buffer (see Recipes)
Permeabilization buffer (see Recipes)
Blocking buffer (see Recipes)
Primary antibodies:
Mouse Anti-E-cadherin (Biosciences, catalog number: 610182)
Rabbit Anti-FOXA2 (Abcam, catalog number: ab108422)
Primary antibody solution (see Recipes)
Secondary antibodies:
Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 555 (ThermoFisher Scientific, catalog number: A31572)
Donkey anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 (ThermoFisher Scientific, catalog number: A21202)
Secondary antibody solution (see Recipes)
Fluorescent probes:
Nuclear staining: Hoechst33342 (Sigma-Aldrich, catalog number: 14533)
Cytoskeleton staining: Phalloidin CruzFluorTM 647 Conjugate (Santa Cruz, catalog number: sc363797)
Nuclear staining solution (see Recipes)
Cytoskeleton staining solution (see Recipes)
Specific for Matrix-embedded 3D model
8-well chambers, #1.5 coverglass (e.g., Cellvis, catalog number: C8-1.5H-N; Ibidi, catalog number: 80807; or NuncTM Lab-TekTM II, Thermo Fisher Scientific, catalog number: 155360)
48-well culture plate (e.g., VWR®, catalog number: 10062-898)
Matrigel® Growth Factor Reduced (GFR) Basement Membrane Matrix, Phenol Red-free, LDEV-free, 10 mL, (Corning, catalog number: 356231) or other suitable, thermo-responsive hydrogels
Dulbecco’s Modified Eagle Medium-F12 mixture (DMEM/F12) with L-glutamine, HEPES, and Phenol Red (GibcoTM, catalog number: 11320032)
TrypLE express (GibcoTM, catalog number: 12604013)
Rock Inhibitor, Y-27632 (Tocris, catalog number: 1254)
Recombinant Human R-Spondin-1 (RSPO-1) (PreproTech, catalog number: 120-38)
Recombinant Human Noggin (NOG) (PreproTech, catalog number: 120-10C)
B27 supplement (50×), minus vitamin A (Life Technologies, catalog number: 12587010)
N-2 supplement (100×) (Life Technologies, catalog number: 17502048)
Insulin Transferrin Selenium (Life Technologies, catalog number: 41400045)
Penicillin/Streptomycin (Life Technologies, catalog number: 15140122)
Nicotinamide (Sigma-Aldrich, catalog number: 73240-100G)
A83-01 (Sigma-Aldrich, catalog number: SML0788-5MG)
N-Acetyl-L-Cystein (Sigma-Aldrich, catalog number: A7250-50G)EGF (R&D Systems, catalog number: 236-EG-01M)
Basic Fibroblast Growth Factor (bFGF) (R&D Systems, catalog number: 233-FB-025)
Fibroblast Growth Factor 10 (FGF-10) (PreproTech, catalog number: 100-26)
P38 Mitogen Activated Protein Kinase inhibitor, SB 202190 (Sigma-Aldrich, catalog number: S7067-5MG)
β Estradiol (Sigma-Aldrich, catalog number: E8750-100MG)
Organoid medium (for endometrial organoids) (see Recipes)
Organoid dissociation solution (see Recipes)
Grace Bio-Labs CoverWellTM perfusion chambers, chamber diam. × thickness: 9 mm × 1.7 mm (Grace Bio-Labs, distributed by Sigma-Aldrich, Merck, catalog number: GBL622205)
Culture plate (e.g., 6-well Sarstedt® cell culture plate, catalog number: 83.3920.500)
Glass coverslips #1.5, 24 × 50 mm (e.g., ThorLabs, catalog number: CG15KH)
Transparent scotch tape
BIOFLOATTM FLEX coating solution for 3D cell culture (faCellitate, catalog number: F202005)
Trypsin/EDTA solution (ThermoFisher Scientific, Invitrogen, catalog number: 15400054)
3D Petri Dish® micro-mold (MicroTissues, Inc., catalog number: 12-256)
Ultra-pure agarose powder (ThermoFisher Scientific, Invitrogen, catalog number: 16500100)
NaCl (Sigma-Aldrich, catalog number: S9888)
Agarose solution (see Recipes)
T25 culture flask (e.g., Sigma-Aldrich, Corning®, catalog number: CLS430639)
Dulbecco’s Modified Eagle Medium (DMEM) (GibcoTM, catalog number: 31053028)
Fetal Bovine Serum (GibcoTM, catalog number: 11533387)
Glutamax (GibcoTM, catalog number: 35050061)
Culture medium (for A549 cells) (see Recipes)
Equipment
Humidified CO2 incubator (ThermoFisher Scientific)
Fridge and freezer (4°C and -20°C)
Table-top centrifuge (ThermoFisher Scientific, Fisher Scientific, catalog number: 12-006-901)
Micropipettes
Leica TCS SP8 dive microscope (Leica microsystems) or other confocal microscope setup with multiphoton excitation
Sterile flow cabinet
Software
Leica application suite (LAS X, Leica microsystems)
LAS X 3D viewer or other image analysis tool such as Fiji/ImageJ (Schindelin et al., 2012)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Van Zundert, I., Maenhoudt, N., De Vriendt, S., Vankelecom, H., Fortuni, B. and Rocha, S. (2022). Fluorescence Imaging of 3D Cell Models with Subcellular Resolution. Bio-protocol 12(14): e4469. DOI: 10.21769/BioProtoc.4469.
分类
发育生物学 > 细胞生长和命运决定
癌症生物学 > 通用技术 > 细胞生物学试验
细胞生物学 > 细胞成像 > 双光子显微镜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link